fecal Calprotectin (fCAL)- An FDA Cleared Gastrointestinal Test to Distinguish IBD from IBS
Calprotectin is a highly-sensitive clinical biomarker that has been shown to be extremely useful as an aid in diagnosis of inflammatory diseases in the gastrointestinal tract.
The BÜHLMANN fCAL® turbo is an in vitro diagnostic assay intended for the quantitative measurement of fecal calprotectin, a neutrophilic protein that is a marker of intestinal mucosal inflammation, in human stool. The BÜHLMANN fCAL® turbo aids in the diagnosis of inflammatory bowel disease (IBD), specifically Crohn’s disease (CD) and ulcerative colitis (UC) and aids in the differentiation of IBD from irritable bowel syndrome (IBS) in conjunction with other laboratory and clinical findings.
Have the courage of your conviction to trust your biomarker…This FDA 510k cleared, non-invasive, sensitive and specific assay is unique in speed, quality, and flexibility. This offers accurate results and reliable information to aid clinicians in selecting patients for further diagnostic procedures.
-
Speed: fastest calprotectin test in the market, time-to-first-result within 10 minutes
-
Quality: standardized against BÜHLMANN fCAL® ELISA, uses highly precise and reproducible PETIA technology
-
Flexibility: can be applied on most open chemistry platforms streamlining your workflow using random access automation
Product Information
| Name | BÜHLMANN fCAL® turbo |
| Method | particle-enhanced turbidimetric immunoassay (PETIA) |
| Sample Type | Fecal extracts |
| Kit Format | 2 reagents; wedge bottles for direct loading on many platforms Calibrators and controls can be provided separately |
| Sample Preparation | CALEX® Cap extracts can be used directly without additional dilution |
| Measuring Range | 30-10,000 µg/g **with retesting |
| Sensitivity (LoQ) | 30 µg/g |
| Sample Volume | ~10 ul Extract (1:500) |
| Time to Result | 10 min (approx.) |
| Applicable | on most chemistry platforms (contact us for information on application notes) |
| Instructions for Use & Other Downloads | see below |
CLINICIANS
Learn about the benefits of the BÜHLMANN fCAL® turbo for your patients.
LABORATORY
Learn about the performance of the BÜHLMANN fCAL® turbo
Learn More
The BÜHLMANN fCAL® turbo sets a new standard in the industry:
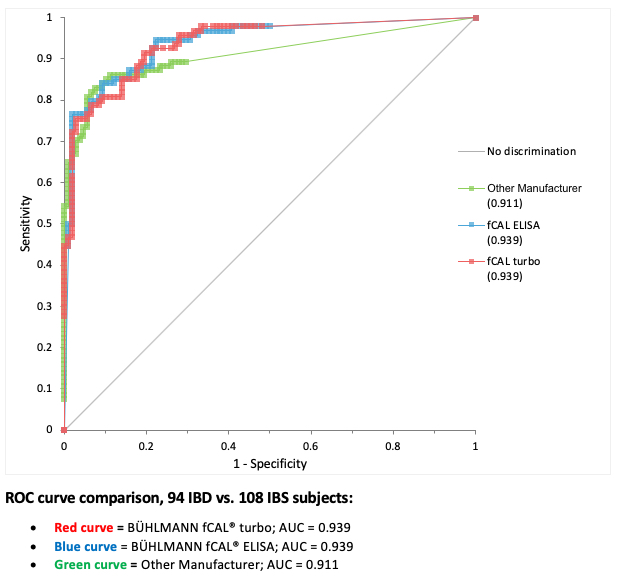
| A clinical study with a total of 248 adult and pediatric patients was performed to assess the ability of the BÜHLMANN fCAL® turbo to discriminate between patients with IBD and other non-inflammatory GI disorders, including IBS. A subset of this population (94 IBD vs 108 IBS) that included only samples that were run with another manufacturer’s assay were used to create the ROC curve below to provide a competitive comparison.
|
 |
Jump to Section:
Workflow: Streamlined Workflow with Total Laboratory Automation (TLA)
BÜHLMANN fCAL® turbo currently has validated IVD application notes for the Roche cobas® c501/5022, Beckman Coulter AU480, Abbott Architect® c400, Siemens ADVIA® XPT and Ortho Vitros® 5600 for use in the U.S.A. Other major chemistry system validation packages already have CE marked validation packages in ROW, or are in process. Please contact BUHLMANN Diagnostics Corp for a complete list of validated applications.
Precision and Reproducibility
Table 1: Reproducibility Study Results- within-run, between-run and between-day variance component estimates
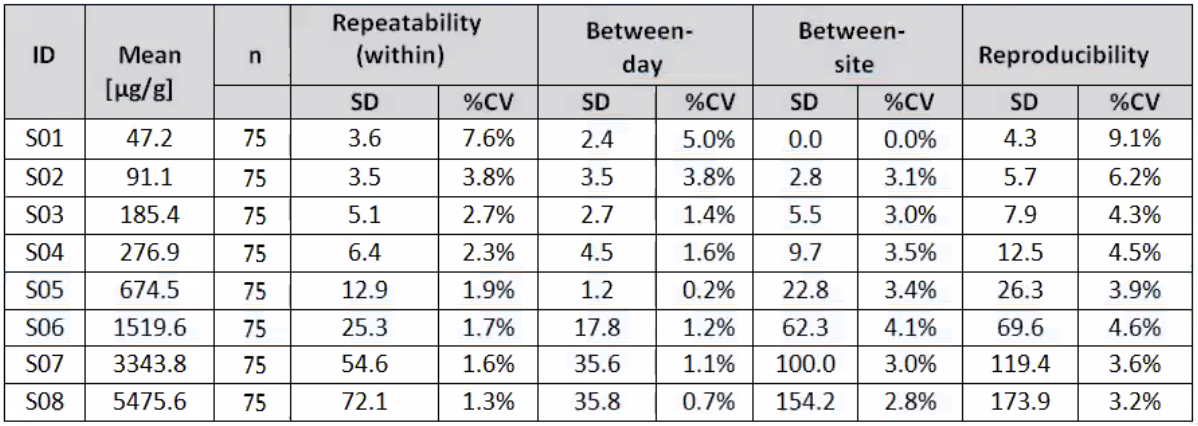
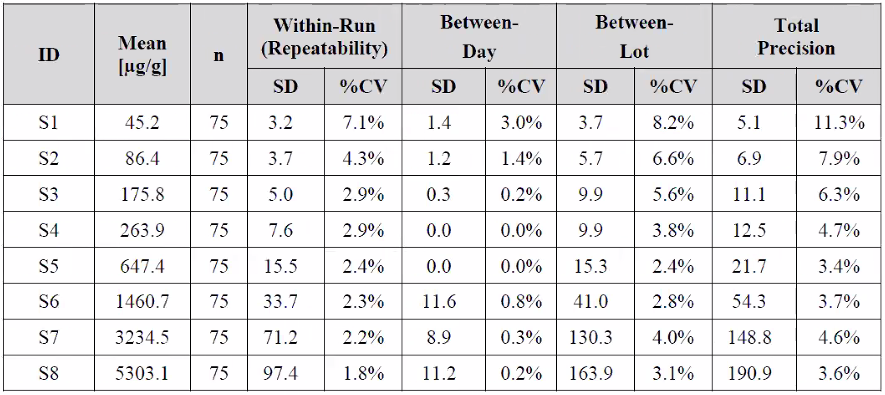
Table 2: Lot to Lot Precision and Reproducibility Results
Linearity
Figure 1: Linearity Data- observed vs. expected results, dilution series #1
y = 2.0 + 1.063 x
r = 0.999
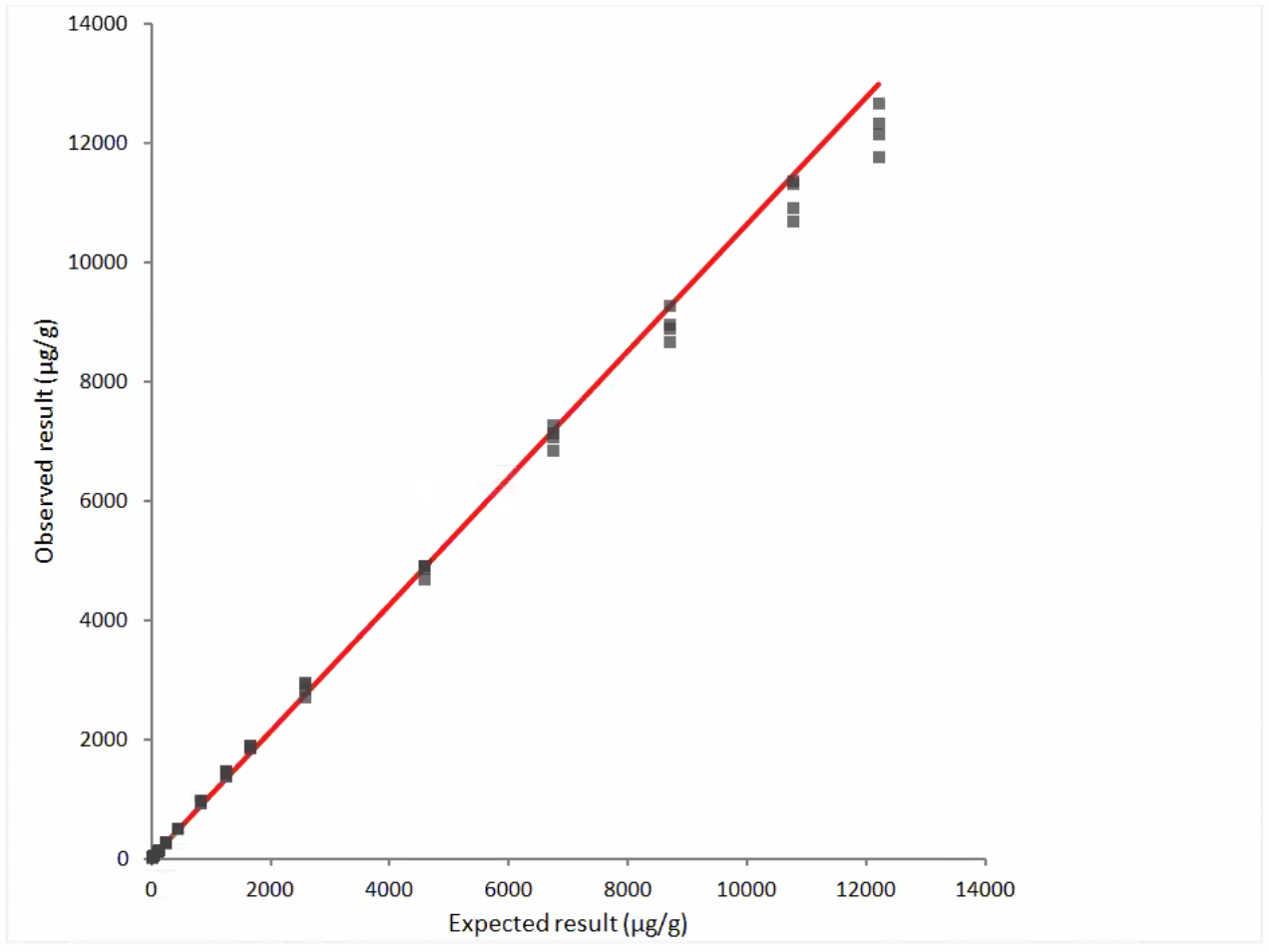
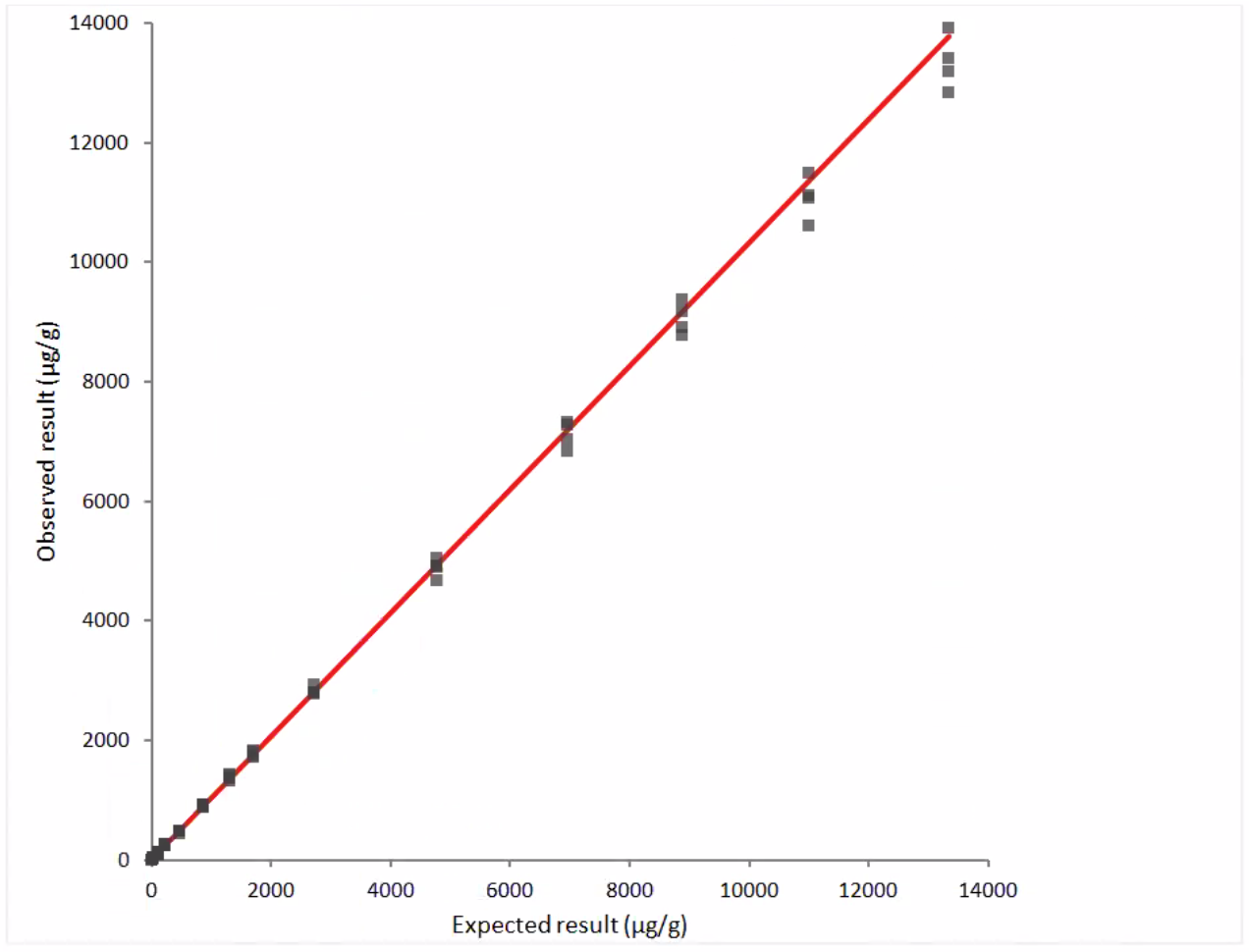
Figure 2: Linearity Data- observed vs. expected results, dilution series #2:
y = 2.3 + 1.033 x
r = 0.999
Instrument Dilution
Table 3: Dilution Study Results – Manual dilution vs automated dilution performed by the instrument for an automated rerun
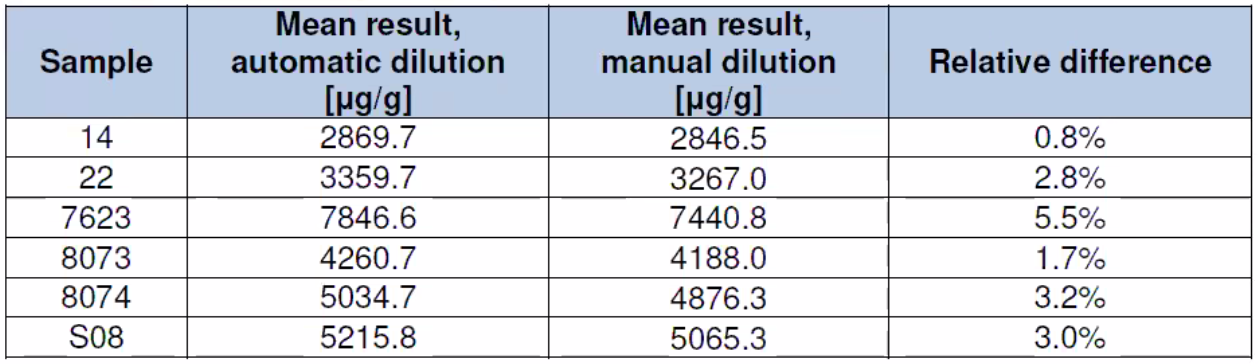
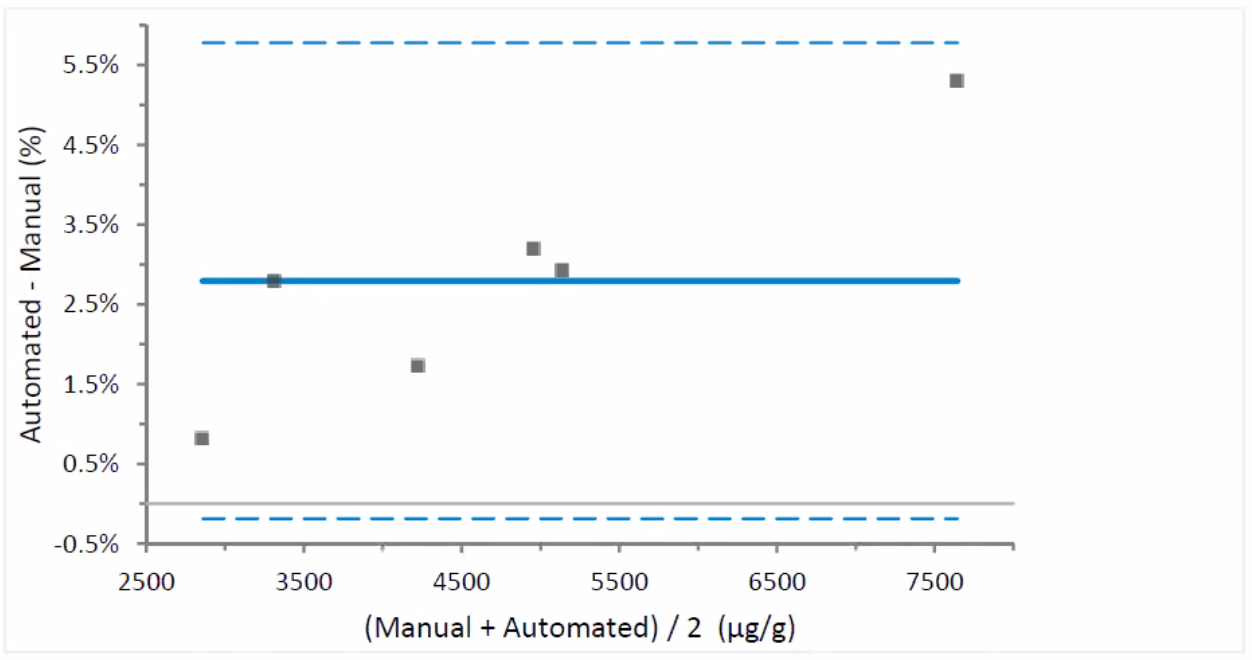
Figure 3: Difference Plot – automated vs. manual dilution
(a mean bias of 2.79% )
Safe stool extract application on clinical chemistry platforms with BÜHLMANN fCAL® turbo and CALEX® Cap.
 Download IFU and Catalog Numbers‡
Download IFU and Catalog Numbers‡
View the appropriate tab below and click on the blue underlined catalog number to view the Instructions for Use.
CALEX® Cap Stool Preparation Device – B-CALEX-C50/ B-CALEX-C200/ B-CALEX-C500
BÜHLMANN fCAL® turbo Components:
All components below are available for sale separately.
Reagent kit B-KCAL-RSET
Calibrator kit B-KCAL-CASET
Control kit B-KCAL-CONSET
Extraction Kit for BÜHLMANN fCAL® tests, if not using CALEX® Cap: B-CAL-EX3 / B-CAL-EX12 (3 x 125ml or 12 x 125ml)
US Application Notes: BÜHLMANN fCAL® turbo currently has the following FDA guideline validated IVD application notes for the the following in the U.S.A:
- Roche cobas® c501/502, Roche cobas® c503, Roche cobas® c701/702,
- Beckman Coulter AU480, Beckman Coulter DxC700AU, Beckman Coulter AU5800,
- Abbott Architect® c400, Abbott Alinity c/ci series,
- Siemens Atellica, Siemens ADVIA® XPT,
- Ortho Vitros® 5600 & 7600 &
- Mindray BS-480
Other major chemistry system validation packages already have CE marked validation packages in ROW, or are in process. Please contact BUHLMANN Diagnostics Corp for a complete list of validated applications. Contact Sales for more information.
BÜHLMANN Products are distributed in Canada by Inter Medico. For more information, email info@inter-medico.com or call 1.800.387.9643.
Stool Preparation Device:
CALEX® Cap Stool Preparation Device – B-CALEX-C50/ B-CALEX-C200/ B-CALEX-C500
CALEX® Cap Stool Preparation Device – B-CALEX-C50/ B-CALEX-C200 / B-CALEX-C500*
BÜHLMANN fCAL® turbo – KK-CAL
This kit contains all the components: extraction kit, reagent kit, calibrator kit, and control kit.
Extraction Kit for BÜHLMANN fCAL® turbo B-CAL-EX3-EX12
Components:
All components below are available for sale separately.
Reagent kit: B-KCAL-RSET
Calibrator kit: B-KCAL-CASET
Control kit: B-KCAL-CONSET
BÜHLMANN fCAL turbo® Clinical Flyer for US
BÜHLMAN fPELA turbo® Clinical Flyer for US
BÜHLMANN CALEX® Cap Medical Flyer for US
BÜHLMANN CALEX® Cap (Fecal Extraction Device) Flyer for US
BÜHLMANN turbo Customer Testimonials
BÜHLMANN fCAL® Calprotectin Key Literature List