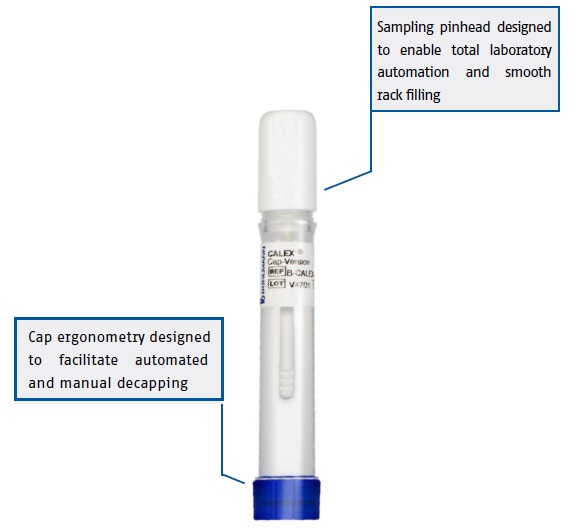
BÜHLMANN fCAL® turbo + CALEX® Cap (K191718 & K232057): FDA 510(k) cleared. For in vitro Diagnostic Use.
CALEX® Cap Stool Preparation Device- for fecal Calprotectin & fecal Pancreatic Elastase
The BÜHLMANN CALEX® Cap is designed to provide convenient and safe stool extraction of multiple analytes. The optimized dilution of stool sample yields maximum extraction efficiency and analyte stability. Calprotectin extracts are stable at room temperature for up to 2 days and up to 15 days at 2-8°C. Pancreatic elastase extracts are stable at room temperature for 8 days and up to 12 days at 2-8°C. CALEX® Cap is the first and only stool preparation device suitable for safe air and land transportation according to IATA 650 (UN3373) regulations. Stool extraction using CALEX® Cap not only offers hygienic stool sampling but also shows very good correlation to the gold standard weighing method for stool extraction.
DESIGN
The design of the CALEX® Cap is fully compatible with laboratory automation including most clinical chemistry analyzers and total lab automation track systems.
Just like the BÜHLMANN fCAL® turbo, this FDA 510k cleared, fecal calprotectin preparation device is unique in speed, quality, and flexibility.
- Designed for TLA to streamline workflow
- Enhanced designed for easy manual or automated operations
- Fast and easy stool extraction
- High correlation to gold standard manual extraction
- Optimal dilution offers excellent efficiency in extraction
Delivering Speed, Quality, Flexibility in Stool Pre-analytics
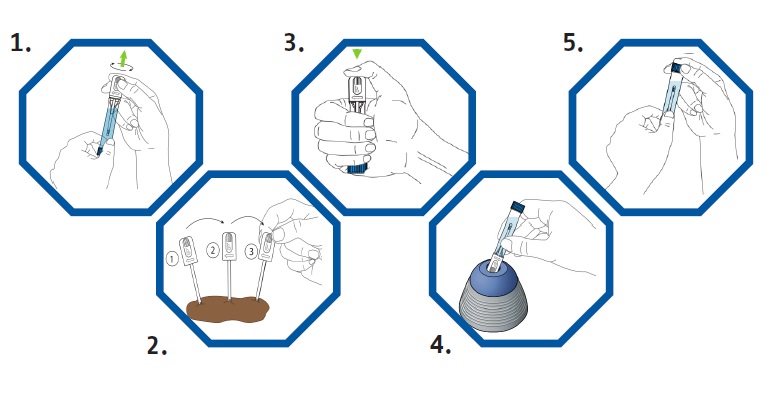
CALEX® Cap is designed to facilitate stool processing by following 5 simple steps, therefore allowing for fast and reliable stool pre-analytics – the key to reliable results.
Time savings in stool pre-analytics are essential to manage increasing sample volumes.
The fecal calprotectin & pancreatic elastase testing workflow is further simplified when using the CALEX® Cap preparation device, reducing hands on time by ~70%
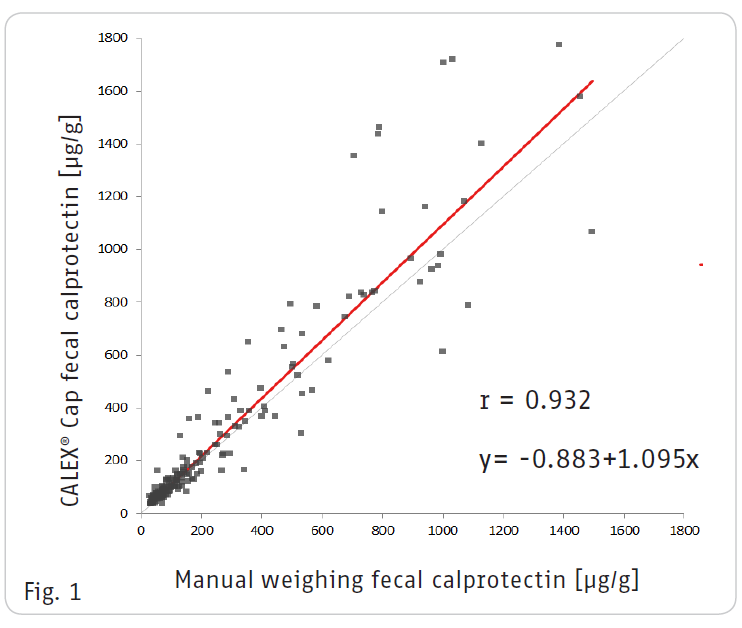
The CALEX® Cap Stool Preparation device combines ease of use and high comparability to the gold standard manual weighing method for the extraction of fecal calprotectin.
A Passing-Bablok regression analysis of 175 patient samples shows an excellent coefficient of correlation of r = 0.932 between the two extraction methods (Fig. 1).
BÜHLMANN fCAL® turbo currently has validated application notes for the Roche c501/502 for use in the U.S. Other major chemistry system validation packages are in process. Please contact BUHLMANN Diagnostics Corp for a complete list of validated applications.
CALEX® Cap is currently FDA 510(k) cleared for use with the BÜHLMANN fCAL® turbo.
Quantum Blue® fCAL is for Research Use Only. Not for use in diagnostic procedures in the US.
CALEX Cap® Stool Preparation Training
CALEX® Cap is currently FDA 510(k) cleared for use with the BÜHLMANN fCAL® turbo.
Quantum Blue® fCAL is for Research Use Only. Not for use in diagnostic procedures in the US.
CALEX Cap® Animated Overview Video
View this brief animated video to see the versatility of the CALEX® Cap stool preparation device, with the BÜHLMANN fCAL® turbo and the BÜHLMANN fPELA® turbo, the random access immunoturbidimetric calprotectin and pancreatic elastase assays, respectively. As well as the Research Use Only Quantum Blue® assay platform..
Watch this animated video to see CALEX® Cap versatility
CALEX Cap® on Clinical Analyzer & Track
The BÜHLMANN fCAL® turbo, fPELA® turbo and complimentary CALEX® Cap fecal preparation device, offers total laboratory automation (TLA) for fecal testing. Simplifying the old cumbersome stool pre-analytics procedure to TLA: from Rack Input Module, to Scanning of Barcode, to Load & Unloading the Centrifuge, to Decapping, to Aspiration of Sample, to Resealing with Foil.
Watch this video and see how CALEX® Cap works on a track system!
BÜHLMANN fCAL® turbo Customer Testimonials
View a compilation of BÜHLMANN fCAL® turbo and CALEX® Cap success stories and testimonials. Find valuable highlights, perspectives, advice and details about the successful applications of this fully automated calprotectin assay workflow from each laboratory.
Click here to see a few independent testimonials!
 Download IFU and Catalog Numbers‡
Download IFU and Catalog Numbers‡
View the appropriate tab and click on the blue underlined catalog number to view the Instructions for Use.
Stool Prep:
- CALEX® Cap Stool Preparation Device– B-CALEX-C50/ B-CALEX-C200/ B-CALEX-C500
Assays:
BÜHLMANN fCAL® turbo –
Components: All components below are available for sale separately.
- Reagent kit B-KCAL-RSET
- Calibrator kit B-KCAL-CASET
- Control kit B-KCAL-CONSET
Extraction Kit for BÜHLMANN fCAL® tests: B-CAL-EX3 / B-CAL-EX12 (3 x 125ml or 12 x 125ml)
BÜHLMANN fPELA® turbo –
Components: All components below are available for sale separately.
- Reagent kit B‐KPELA‐RSET
- Calibrator kit B‐KPELA‐CASET
- Control kit B‐KPELA‐CONSET
Extraction Kit for BÜHLMANN fCAL® tests, if not using CALEX® Cap: B-CAL-EX3 / B-CAL-EX12 (3 x 125ml or 12 x 125ml)
BÜHLMANN Products are distributed in Canada by Inter Medico. For more information, email info@inter-medico.com or call 1.800.387.9643.
Extraction Device:
CALEX® Cap Stool Preparation Device – B-CALEX-C50/ B-CALEX-C200 / B-CALEX-C500*
CALEX® Cap Stool Preparation Device – B-CALEX-C50/ B-CALEX-C200 / B-CALEX-C500*
* Multilingual [EN, DE, FR, IT, ES]
ELISA:
BÜHLMANN fCAL®ELISA – EK-CAL /CAL2
ELISA Kit Sizes: EK-CAL: 96 wells (1 plate), EK-CAL2: 192 tests (2 plate)
EK-CAL2-WEX 192 tests (without extraction buffer for use with CALEX®)
Automation:
BÜHLMANN fCAL® turbo – KK-CAL
This kit contains all the components: extraction kit, reagent kit, calibrator kit, and control kit.
Components:
All components below are available for sale separately.
Reagent kit: B-KCAL-RSET
Calibrator kit: B-KCAL-CASET
Control kit: B-KCAL-CONSET
Watch Calprotectin Video (CDHF)
BÜHLMANN fPELA® turbo – KK-PELA
Components: All components below are available for sale separately.
Reagent kit B‐KPELA‐RSET
Calibrator kit B‐KPELA‐CASET
Control kit B‐KPELA‐CONSET
Extraction Kit for BÜHLMANN fCAL® tests, if not using CALEX® Cap: B-CAL-EX3 / B-CAL-EX12 (3 x 125ml or 12 x 125ml)
BÜHLMANN fCAL turbo® Clinical Flyer for US
BÜHLMAN fPELA turbo® Clinical Flyer for US
BÜHLMANN CALEX® Cap Medical Flyer for US
BÜHLMANN CALEX® Cap (Fecal Extraction Device) Flyer for US
BÜHLMANN turbo Customer Testimonials
BÜHLMANN fCAL® Calprotectin Key Literature List