- Products & Solutions
- FDA IVD
- About Us
- News & Events
- Contact
- Additional Sites
BUHLMANN News

Visit BUHLMANN at ADLM 2025
Product Highlights IVD Assays RUO Solutions ADLM 2025 Info Product Highlights Gastroenterology We offer a comprehensive gastroenterology testing portfolio, featuring ...

Product Highlights – ACE Kinetic Enzymatic Assay
BÜHLMANN ACE Kinetic Enzymatic Assay Unique in Speed, Quality, Flexibility Automated Assay for use on Multiple Clinical Chemistry Platforms ACE ...

Visit BUHLMANN at DDW 2025
Product Highlights DDW 2025 Venue Buhlmann Team Product Highlights We offer a comprehensive gastroenterology testing portfolio, featuring a range of ...

BÜHLMANN Laboratories AG Announces Worldwide Collaboration with Beckman Coulter for BÜHLMANN fCAL turbo and fPELA turbo Assays
Amherst NH, September 2024- BÜHLMANN Laboratories AG, a leading manufacturer of specialty, high-quality in-vitro diagnostic and research assays, is pleased ...

AMLI 2024 Recap for BUHLMANN Diagnostics Corp
Key Highlights BUHLMANN Diagnostics Corp proudly participated in the 37th Annual Meeting of the Association of Medical Laboratory Immunologists (AMLI) ...

BUHLMANN Diagnostics Corp’s ADLM 2024 Recap
Key Highlights We are thrilled to share the highlights of our successful participation in the Association for Diagnostics and Laboratory ...

BUHLMANN Diagnostics Corp Recap from DDW 2024
Key Highlights BUHLMANN Diagnostics Corp is excited to share highlights from our participation at Digestive Disease Week (DDW) 2024, held ...
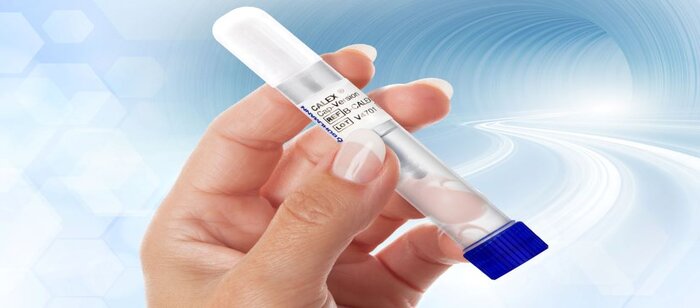
CALEX Cap Updates- Extended Stability, Training Video & CALEX Cap 4.0
BÜHLMANN fCAL® turbo + CALEX® Cap (K191718 & K232057): FDA 510(k) cleared. For in vitro Diagnostic Use. BÜHLMANN fPELA® turbo is FDA ...




