Product Highlights
We offer a comprehensive gastroenterology testing portfolio, featuring a range of assays for fecal calprotectin (fCAL), pancreatic elastase, and therapeutic drug monitoring (TDM). Our fCAL testing lineup includes the FDA 510(k)-cleared BÜHLMANN fCAL® ELISA, BÜHLMANN fCAL® turbo, and CALEX® Cap, as well as the Research Use Only (RUO) Quantum Blue® fCAL rapid tests. Additionally, we offer the FDA-exempt BÜHLMANN fPELA® turbo assay for pancreatic elastase and RUO Quantum Blue® IFX & ADL TDM and antibody rapid tests.
Fecal calprotectin and pancreatic elastase are often ordered together to assess GI function
Our diagnostic solutions deliver comprehensive insights (1 extract, 2 biomarkers), offering simultaneous biomarker testing. Designed for speed, our assays rank among the fastest available, enhancing efficiency and supporting total lab automation. Backed by decades of expertise and our patented CALEX® technology, we ensure proven accuracy and a louder alarm for inflammation. With flexible scalability, our solutions integrate seamlessly into various lab workflows, supporting automation and multi-analyte testing.
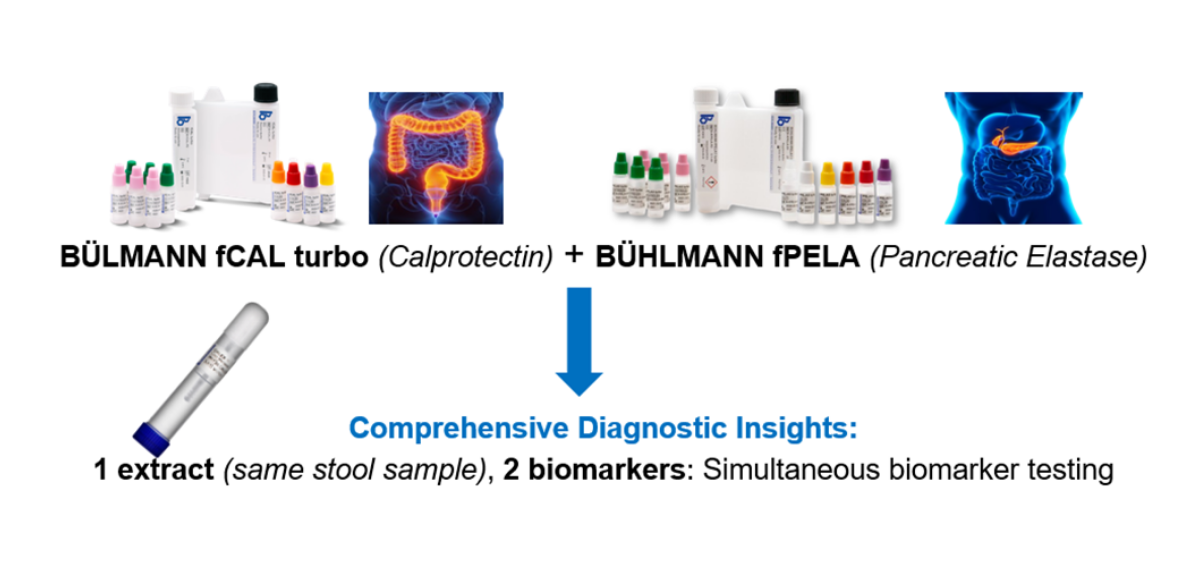
BUHLMANN fCAL® turbo

The BÜHLMANN fCAL® turbo is an in vitro diagnostic assay intended for the quantitative measurement of fecal calprotectin, a neutrophilic protein that is a marker of intestinal mucosal inflammation, in human stool. This FDA 510k cleared, non-invasive, sensitive and specific assay is unique in speed, quality, and flexibility. This offers accurate results and reliable information to aid clinicians in selecting patients for further diagnostic procedures.
- Speed: ranked among the fastest calprotectin tests in the market, time-to-first-result within 10 minutes
- Quality: standardized against BÜHLMANN fCAL® ELISA, uses highly precise and reproducible PETIA technology
- Flexibility: can be applied on most open chemistry platforms streamlining your workflow using random access automation
(US:BÜHLMANN fCAL® turbo (K190784): FDA 510(k) cleared. For in vitro Diagnostic Use.BÜHLMANN fCAL® turbo + CALEX® Cap (K191718 & K232057): FDA 510(k) cleared. For in vitro Diagnostic Use.CPT Code: Calprotectin – 83993)
BUHLMANN fPELA® turbo

The BÜHLMANN fPELA® turbo is an in vitro diagnostic test for the quantitative determination of pancreatic elastase in human fecal extracts. The results can be used as an aid to determination of exocrine pancreatic insufficiency in patients suffering from conditions such as chronic pancreatitis and in conjunction with other laboratory and clinical findings. The BÜHLMANN fPELA® turbo assay is intended to be run on clinical chemistry analyzers. For laboratory use only.
- Speed: fast pancreatic test, time-to-first-result within 10 minutes
- Quality: high correlation to the manual gold standard reference method, specific to human enzymatic isoforms, and uses highly precise and reproducible PETIA technology
- Flexibility: can be applied on most open chemistry platforms (in the same fecal extract used for calprotectin testing) streamlining your workflow using random access automation
(US:BÜHLMANN fPELA® turbo: FDA 510(k) Exempt. For in vitro Diagnostic Use. CPT Code: Pancreatic Elastase– 123234)
CALEX® Cap
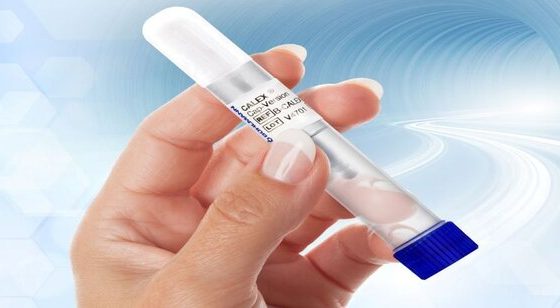
CALEX® Cap Stool Preparation Device- for fecal Calprotectin & fecal Pancreatic Elastase
The BÜHLMANN CALEX® Cap is designed to provide convenient and safe stool extraction of multiple analytes. The optimized dilution of stool sample yields maximum extraction efficiency and analyte stability. Calprotectin extracts are stable at room temperature for up to 2 days and up to 15 days at 2-8°C. Pancreatic elastase extracts are stable at room temperature for 8 days and up to 12 days at 2-8°C.
Venue Information








