

Slide


Pancreatic Elastase
BÜHLMANN fPELA® turbo
BÜHLMANN fPELA® turbo
_____
Random Access Turbidimetric Immunoassay
Use with CALEX® Cap for
Unmatched Efficiency & Analyte Stability
fCAL-fPELA-turbo-BDC

The BÜHLMANN fPELA® turbo is a test for the quantitative determination of pancreatic elastase in human fecal extracts and is intended to be run on clinical chemistry analyzers.
This test is a particle enhanced turbidimetric immunoassay (PETIA) assay developed to be run directly with stool extracts derived from the CALEX® Cap extraction device. This device is pre-filled with unique extraction buffer designed for fecal extraction of pancreatic elastase and calprotectin, allowing for quantification of both analytes at the same time.
Fecal pancreatic elastase is a well-established biomarker to detect exocrine pancreatic insufficiency in patients suffering from conditions such as chronic pancreatitis. The concentration of pancreatic elastase levels in feces reflects the level of pancreatic output and correlates with the output of other pancreatic enzymes such as lipase, amylase, and trypsin.
This assay is a non-invasive, sensitive and specific assay that is unique in speed, quality, and flexibility. This offers accurate results and reliable information to aid clinicians in selecting patients for further diagnostic procedures.
Speed:
fast pancreatic test, time-to-first-result within 10 minutes
Quality:
high correlation to the manual gold standard reference method, specific to human enzymatic isoforms, and uses highly precise and reproducible PETIA technology
Flexibility
can be applied on most open chemistry platforms (in the same fecal extract used for calprotectin testing) streamlining your workflow using random access automation
“The analysis with the cobas® is more flexible, more cost efficient and faster than classical microplate based ELISA assays.” A report from Dr. Jörg Oliver Thumfart.
| Name | BÜHLMANN fPELA® turbo |
| Method | particle-enhanced turbidimetric immunoassay (PETIA) |
| Sample Type | Fecal extracts |
| Kit Format | 2 reagents (R1/ R2) Calibrators and controls provided separately |
| Sample Preparation | CALEX® Cap extracts ready to use without dilution |
| Measuring Range | 10-5000 µg/g |
| Sensitivity (LoQ) | 5.7 µg/g |
| Sample Volume | ~10 ul centrifuged fecal Extract (1:500) |
| Time to Result | ~10 min CALEX® Extraction ~20 min |
| Applicable | on most chemistry platforms (contact us for information on application notes) |
| Instructions for Use & Other Downloads | see below |
CLINICIANS
Learn about the benefits of the BÜHLMANN fPELA® turbo for your patients.
LABORATORY
Learn about the performance of the BÜHLMANN fPELA® turbo
Testing was performed on Roche c501, Beckman Coulter AU480, and Mindray BS380 instruments. Six (6) stool specimen extracts were assayed.
Table 1: Reproducibility Study Results- within-run, between-day and between-instrument variance
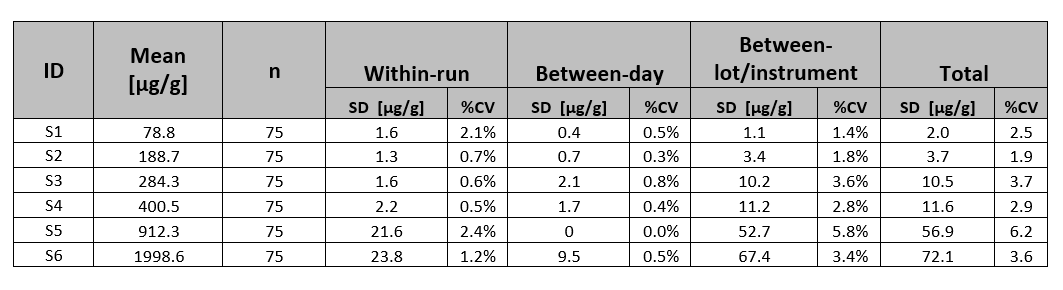
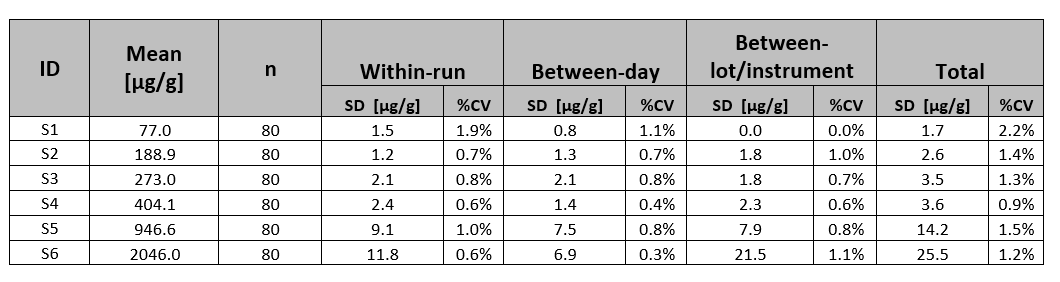
Table 2: Within-Laboratory Precision and Reproducibility Results
Linearity range: 3.4 to 5024.2 µg/g
The linear range of the BÜHLMANN fPELA® turbo was determined according to the CLSI guideline EP06-A. Samples with a concentration of over 500 μg/g were diluted automatically 1:10 by the analyzer. A maximum deviation from linearity of 10% or 10 µg/g, for samples below 100 µg/g, was allowed.
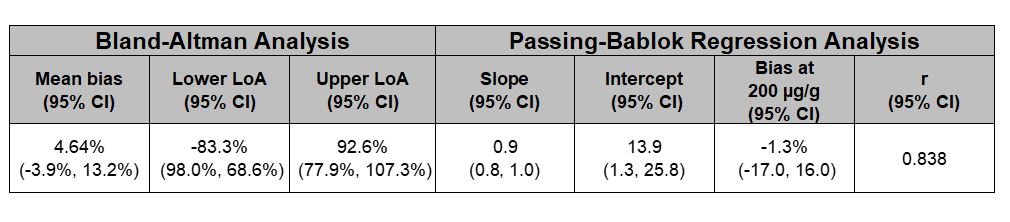
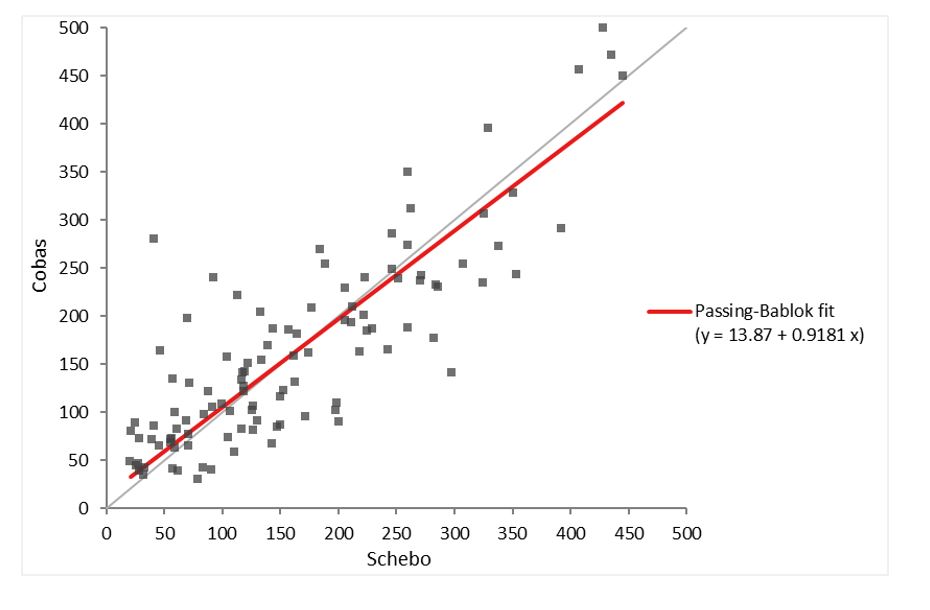
Table 3: Method Comparison Study Results – BÜHLMANN fPELA® turbo vs ScheBo® Pancreatic Elastase 1TM
The method comparison study was performed according to the CLSI guideline EP09-A3. One hundred and eight (108) samples were measured using three lots of BÜHLMANN fPELA® turbo over four days. Mean reference values, with elastase concentrations falling within the measuring range of 15– 500 µg/g µg/g, were established with two lots of the ScheBo® Pancreatic Elastase 1TM test. Bias was determined using Passing-Bablok linear regression and Bland-Altman analysis.
| BÜHLMANN fPELA® turbo |
|
||||
| > 200 μg/g | 100-200 μg/g | < 100 μg/g | TOTAL | ||
| > 200 μg/g | 37 | 9 | 2 | 48 | |
| 100-200 μg/g | 5 | 22 | 9 | 36 | |
| < 100 μg/g | 1 | 8 | 37 | 46 | |
| Total | 43 | 39 | 48 | 130 | |
Extraction Device:
CALEX® Cap Stool Preparation Device – B-CALEX-C50/ B-CALEX-C200/ B-CALEX-C500
BÜHLMANN fPELA® turbo Components:
All components below are available for sale separately.
Reagent kit B‐KPELA‐RSET
Calibrator kit B‐KPELA‐CASET
Control kit B‐KPELA‐CONSET
Extraction Kit for BÜHLMANN fPELA® tests, if not using CALEX® Cap: B-CAL-EX3 / B-CAL-EX12 (3 x 125ml or 12 x 125ml)
US Application Notes: BÜHLMANN fPELA® turbo currently has the following FDA guideline validated IVD application notes, for the following in the USA:
Other major chemistry system validation packages already have CE marked validation packages in ROW, or are in process. Please contact BUHLMANN Diagnostics Corp for a complete list of validated applications. Contact Sales for more information.
BÜHLMANN Products are distributed in Canada by Inter Medico. For more information, email info@inter-medico.com or call 1.800.387.9643.
Extraction Device:
CALEX® Cap Stool Preparation Device – B-CALEX-C50/ B-CALEX-C200 / B-CALEX-C500*
BÜHLMANN fCAL® turbo – KK-PELA
This kit contains all the components: extraction kit, reagent kit, calibrator kit, and control kit.
Components:
All components below are available for sale separately.
Reagent kit: B-KPELA-RSET
Calibrator kit: B-KPELA-CASET
Control kit: B-KPELA-CONSET
BÜHLMAN fPELA turbo® Clinical Flyer for US
BÜHLMANN fCAL turbo® Clinical Flyer for US
BÜHLMANN CALEX® Cap Medical Flyer for US
BÜHLMANN CALEX® Cap (Fecal Extraction Device) Flyer for US
BÜHLMANN turbo Customer Testimonials
BÜHLMANN fCAL® Calprotectin Key Literature List