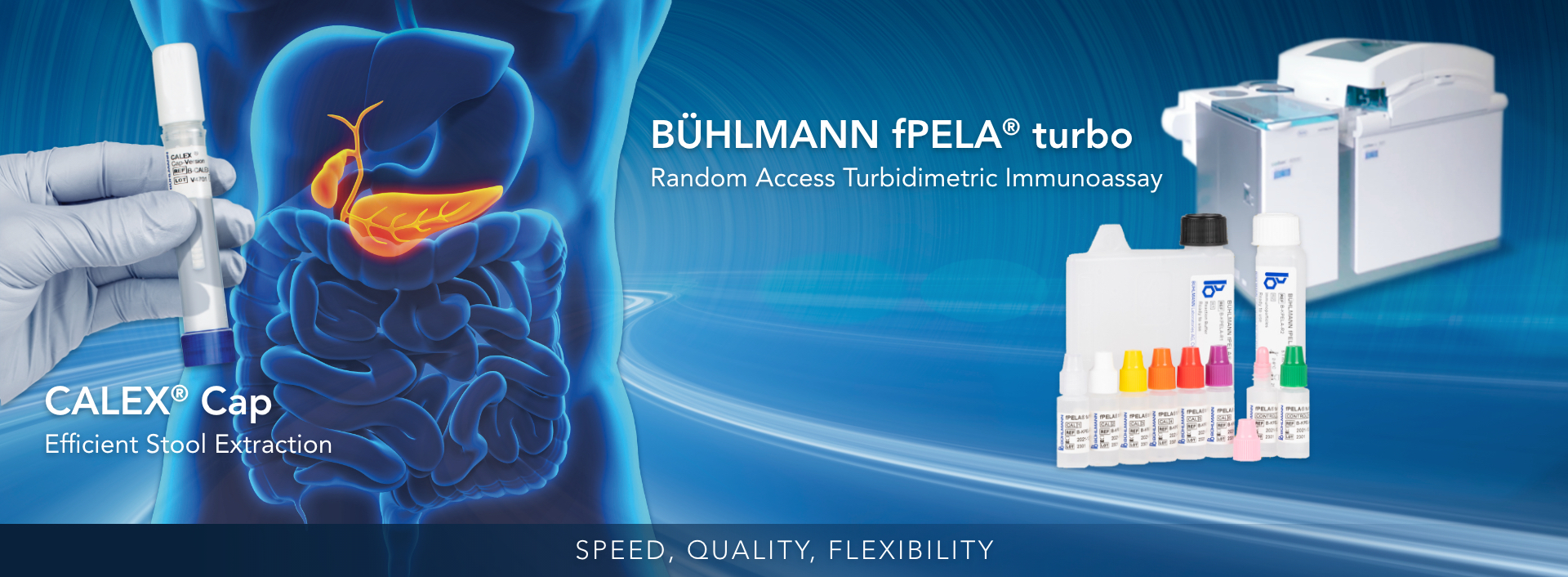
BÜHLMANN fCAL® turbo or BÜHLMANN fPELA® turbo
BÜHLMANN fCAL® turbo or BÜHLMANN fPELA® turbo currently have validated IVD application notes for use in the United States on the clinical chemistry analyzers listed below. These allow for the FDA 510(k) cleared fecal calprotectin or FDA Exempt fecal pancreatic elastase to be tested on the Roche cobas® c501/502, Ortho Vitros® 5600 & 7600, Beckman Coulter AU480, Abbott Alinity or Architect® c4000 and Siemens Atellica or ADVIA® XPT analyzers, Mindray BS-480 and Binding Site Optilite®, to name a few. Other major chemistry system validation packages already have CE marked validation packages for use in ROW, or are in process. Please contact BUHLMANN Diagnostics Corp for a complete list of validated applications.
- Roche cobas® c501/502, c701/702 & Pro Module c503
- Ortho Vitros® 5600 & 7600
- Beckman Coulter AU480, DxC700AU & AU series
- Abbott Alinity, Architect® c4000, & c-series
- Siemens ADVIA® XPT & Atellica
- Thermo Scientific Indiko
- BioSystems BA200
- Binding Site Optilite®
- Mindray BS-480
BÜHLMANN fCAL® turbo
Intended Use:
The BÜHLMANN fCAL® turbo is an in vitro diagnostic assay intended for the quantitative measurement of fecal calprotectin, a neutrophilic protein that is a marker of intestinal mucosal inflammation, in human stool. The BÜHLMANN fCAL® turbo aids in the diagnosis of inflammatory bowel disease (IBD), specifically Crohn’s disease (CD) and ulcerative colitis (UC) and aids in the differentiation of IBD from irritable bowel syndrome (IBS) in conjunction with other laboratory and clinical findings.
An FDA-Cleared Gastrointestinal Test to Distinguish IBD from IBS
This FDA 510(k) cleared, non-invasive, sensitive and specific calprotectin assay is unique in speed, quality, and flexibility. This offers accurate results and reliable information to aid clinicians in selecting patients for further diagnostic procedures.
- Speed: fastest calprotectin test in the market, time-to-first-result within 10 minutes
- Quality: standardized against BÜHLMANN fCAL® ELISA, uses highly precise and reproducible PETIA technology
- Flexibility: can be applied on most open chemistry platforms streamlining your workflow using random access automation
BÜHLMANN fPELA® turbo
Intended Use:
The BÜHLMANN fPELA® turbo is an in vitro diagnostic test for the quantitative determination of pancreatic elastase in human fecal extracts. The results can be used as an aid to determination of exocrine pancreatic insufficiency in patients suffering from conditions such as chronic pancreatitis and in conjunction with other laboratory and clinical findings. The BÜHLMANN fPELA® turbo assay is intended to be run on clinical chemistry analyzers. For laboratory use only.
A Gastrointestinal Test to Detect Endocrine Pancreatic Insufficiency
The FDA Exempt BÜHLMANN fPELA® turbo is a test for the quantitative determination of pancreatic elastase in human fecal extracts and is intended to be run on clinical chemistry analyzers.
This test is a particle enhanced turbidimetric immunoassay (PETIA) assay developed to be run directly with stool extracts derived from the CALEX® Cap Stool Preparation device. This device is pre-filled with unique extraction buffer designed for fecal extraction of pancreatic elastase and calprotectin, allowing for quantification of both analytes at the same time.
- Speed: fast pancreatic test, time-to-first-result within 10 minutes
- Quality: high correlation to the manual gold standard reference method, specific to human enzymatic isoforms, and uses highly precise and reproducible PETIA technology
- Flexibility: can be applied on most open chemistry platforms (in the same fecal extract used for calprotectin testing) streamlining your workflow using random access automation
BÜHLMANN CALEX® Cap
Intended Use:
The BÜHLMANN CALEX® Cap is a single use tube intended for the preparation of human stool samples to be used with the BÜHLMANN fCAL® turbo and BÜHLMANN fPELA® turbo.
CALEX® Cap Stool Preparation Device
The BÜHLMANN CALEX® Cap has been introduced to fulfill the requirements of convenient and safe stool extraction. The optimized dilution of stool sample yields maximum extraction efficiency while offering a stability of the extracts for up to 3.5 days (84 hours) or up to 8 days at 2-8°C, for Calprotectin and Elastase, respectively. CALEX® Cap is the first and only stool preparation device suitable for safe air and land transportation according to IATA 650 (UN3373) regulations. The extraction using CALEX® Cap not only offers hygienic stool sampling but also shows very good correlation to gold standard weighing method for stool extraction and is therefore characterized by high reliability.
Delivering Speed, Quality, Flexibility in Stool Pre-analytics
Delivering Speed, Quality, Flexibility in Stool Pre-analytics
- Speed: CALEX® Cap is designed to facilitate stool extraction by following 5 simple steps, therefore allowing for fast and reliable stool pre-analytics – the key to reliable results.
- Quality: The CALEX® Cap stool preparation device combines ease of use and high comparability to the common manual weighing method for the extraction of fecal calprotectin.
- Flexibility: Watch the CALEX® Cap in action for Total Laboratory Automation (TLA)
Related Videos
Other major chemistry system validation packages already have CE marked validation packages in ROW, or are in process. Please contact BUHLMANN Diagnostics Corp for a complete list of validated applications.
Success Stories:
Find our listing of success stories identifying how simple set up was for use on your analyzer:
https://buhlmannlabs.com/category/success-stories/
Learn more about extraction with CALEX® Cap & see how you can reduce hands-on-time by 70%.