AACC 2023 Assays Offerings
Assays Offerings: BUHLMANN Diagnostic Corp (BDC), offers the broadest fecal calprotectin (fCAL) product range in the industry, available in the United States, including FDA cleared and Research Use Only (RUO) kits. These fCAL related tests include FDA 510(k) cleared BÜHLMANN fCAL® ELISA, BÜHLMANN fCAL® turbo & CALEX® Cap; FDA exempt BÜHLMANN fPELA® turbo (Pancreatic Elastase); Tests available as RUO include: IBDoc®, Quantum Blue® fCAL rapid tests and Quantum Blue® IFX & ADL TDM and Antibody rapid tests. Beyond calprotectin, BDC offers unique assays for Cellular Allergy (Flow CAST®), Neuroimmunology (MAG, SPGP, Gangliosides) and Clinical Chemistry.
Visit buhlmannlabs.com or email sales@buhlmannlabs.com for more information.
AACC 2023 Annual Scientific Meeting & Clinical Lab Expo
July 23-27
Anaheim Convention Center / Anaheim, CA
Booth: 1918
Visit our booth at AACC 2023 to get an update on our Calprotectin, Pancreatic Elastase & CALEX Cap® automated assays, or learn more about evolving in-vitro allergy testing, in a vial.
The BÜHLMANN fCAL® turbo is an in vitro diagnostic assay intended for the quantitative measurement of fecal calprotectin, a neutrophilic protein that is a marker of intestinal mucosal inflammation, in human stool. This FDA 510k cleared, non-invasive, sensitive and specific assay is unique in speed, quality, and flexibility. This offers accurate results and reliable information to aid clinicians in selecting patients for further diagnostic procedures.
Because Accuracy is What Matters
- Robust: accurate, precise & efficient assay to instill confidence
- Validated: in use worldwide providing over 1 million reliable patient results a year
- Published: highly referenced, >100 peer reviewed scientific articles
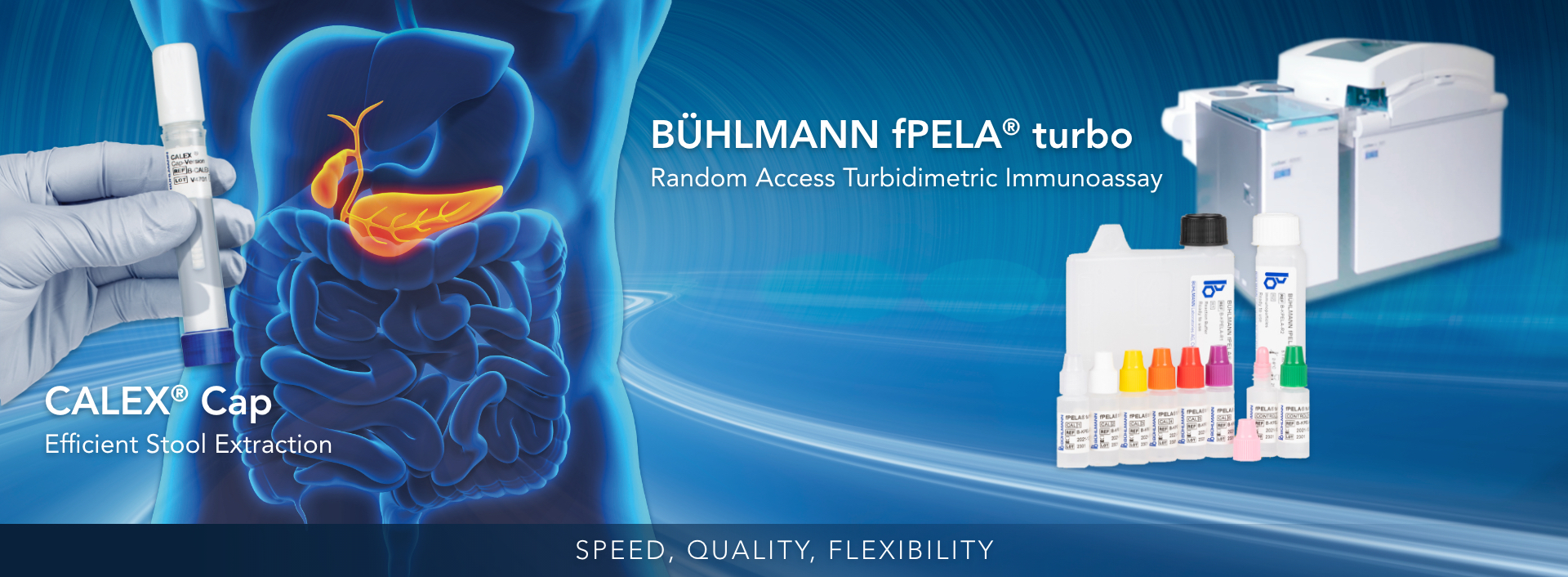
The BÜHLMANN fPELA® turbo is a test for the quantitative determination of pancreatic elastase in human fecal extracts and is intended to be run on clinical chemistry analyzers. Fecal pancreatic elastase is a well-established biomarker to detect exocrine pancreatic insufficiency in patients suffering from conditions such as chronic pancreatitis. The concentration of pancreatic elastase levels in feces reflects the level of pancreatic output and correlates with the output of other pancreatic enzymes such as lipase, amylase, and trypsin.
This assay is a non-invasive, sensitive and specific assay that is unique in speed, quality, and flexibility. This offers accurate results and reliable information to aid clinicians in selecting patients for further diagnostic procedures.
- Speed: fast pancreatic test, time-to-first-result within 10 minutes
- Quality: high correlation to the manual gold standard reference method, specific to human enzymatic isoforms, and uses highly precise and reproducible PETIA technology
- Flexibility: can be applied on most open chemistry platforms (in the same fecal extract used for calprotectin testing) streamlining your workflow using random access automation