
Assay Offerings
Assay Offerings: We offer the broadest fecal calprotectin (fCAL) product range in the industry, available in the United States, including FDA cleared and Research Use Only (RUO) kits. These fCAL related tests include FDA 510(k) cleared BÜHLMANN fCAL® ELISA, BÜHLMANN fCAL® turbo & CALEX® Cap; FDA exempt BÜHLMANN fPELA® turbo (Pancreatic Elastase); Tests available as RUO include: IBDoc®, Quantum Blue® fCAL rapid tests and Quantum Blue® IFX & ADL TDM and Antibody rapid tests. Also included in our RUO portfolio are unique assays for Cellular Allergy, Neuroimmunology, Clinical Chemistry and Chronobiology.
Visit buhlmannlabs.com or email sales@buhlmannlabs.com for more information.
DDW 2024 – Digestive Disease Week 2024
May 18-21, 2024
Washington, D.C.
Booth: 2128
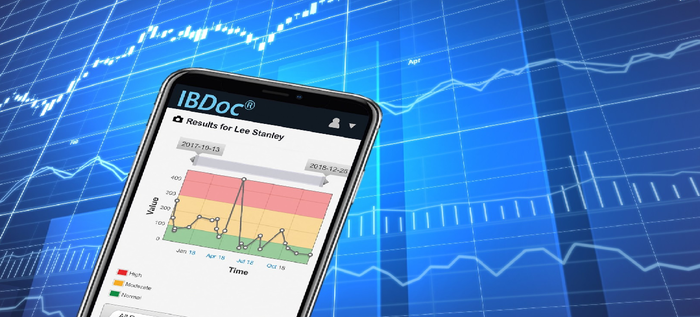
IBDoc®, the first in-vitro diagnostic POC and home testing device to measure the inflammatory marker, fecal calprotectin.
BÜHLMANN IBDoc® is an in vitro diagnostic immunoassay for the quantitative determination of fecal calprotectin in human stool. The results of the assay are analyzed by a downloadable smartphone application. IBDoc® is intended as an aid to the determination of the inflammatory status of the patient’s intestinal mucosa for inflammatory bowel disease (e.g. Crohn’s Disease and Ulcerative Colitis) monitoring. IBDoc® is an assay developed for self-testing / home use by trained patients ages 12 and above that are under the care of a healthcare practitioner. The test may also be used in a near-patient or laboratory setting.
Health Canada Licence: 98903, Device class: 3
BÜHLMANN IBDoc® is not available for sale in the US.

The BÜHLMANN fCAL® turbo is an in vitro diagnostic assay intended for the quantitative measurement of fecal calprotectin, a neutrophilic protein that is a marker of intestinal mucosal inflammation, in human stool. This FDA 510k cleared, non-invasive, sensitive and specific assay is unique in speed, quality, and flexibility. This offers accurate results and reliable information to aid clinicians in selecting patients for further diagnostic procedures.
Because Accuracy is What Matters
Robust: accurate, precise & efficient assay to instill confidence
Validated: in use worldwide providing over 1 million reliable patient results a year
Published: highly referenced, >100 peer reviewed scientific articles

Quantum Blue® platform offering rapid and quantitative results for serum calprotectin, CRP, serum IFX trough levels, and serum ADL trough levels (Additional trough level assays coming soon!)
Health Canada Licenses:
Quantum Blue® IFX: 98838
Quantum Blue® ADL: 101776
Quantum Blue® IFX and Quantum Blue® ADL is for Research Use Only in the US. Not for use in diagnostic procedures.






