New JACI paper from Patil et al:
Food allergy is a growing public health burden in children and adults all over the world. In some countries, up to 10% of infants are affected by food allergies (Loh et al., 2018). Relatively few foods like cow milk, egg, wheat, soy, nuts, fish, and shellfish account for the majority of allergies. Nevertheless, every food at any age can cause allergic reactions, from mild symptoms such as itchiness up to serious reactions including anaphylaxis.
Diagnosis of food allergy can be quite cumbersome. Screening tests like skin prick tests and serology determination of IgE antibodies specific to foods are the only measure of the sensitization to foods supporting clinicians in the diagnostic workflow. But clinical cut-offs for allergy are critical to be determined.
While the gold standard for diagnosis remains the oral food challenge, a novel diagnostics such as the basophil activation test (BAT), which is a standardized in vitro induction of allergic reaction, is reported to reproduce a patient’s allergic reaction in a safe blood test (Scott et al., 2017). At the moment, there is no cure for food allergies and disease management remains largely dependent on allergen avoidance and treating emergency cases of severe reactions and anaphylaxis.
New hope comes from pharmaceutical immunotherapy based on the concept of exposing patients to increasing amounts of allergen in an attempt to change the immune system’s response. Although this approach has not been demonstrated to cure a specific allergy, it has the goal of achieving a sustained clinical desensitization to the allergen itself. In February 2020, the FDA approved PALFORZIA as the first drug for oral immunotherapy to treat patients having a confirmed diagnosis of peanut allergy.
In this context, we interviewed Michael Schneider, Vice President of Product Development at BÜHLMANN Laboratories AG who is part of an international team publishing an outstanding research paper on “Early decrease in basophil sensitivity to Ara h 2 precedes sustained unresponsiveness after peanut oral immunotherapy” in the top rated Journal of Allergy and Clinical Immunology.
Interview:
Hello Mr. Schneider, you recently published an article with the focus on peanut oral immunotherapy. Why is this topic so important?
At the moment there is no cure for food allergy. Immunotherapy is the new hope for patients to deal with severe allergic reactions. Recently, the first immunotherapy product PALFORZIA received approval by the FDA for the treatment of confirmed peanut allergy. PALFORZIA is a peanut allergen powder used to expose children to increasing doses of peanuts to mitigate allergic reactions. Hopefully, new products for additional allergens will become available soon. Please note, that clinical trials demonstrated that immunotherapy does not cure allergic reactions but reduces the severity including anaphylactic reactions which may occur upon accidental exposure to the allergen.
What do you addressed in your research paper?
In collaboration with the clinical team of Dr. Wayne Shreffler and Dr. Sarita Patil from Massachusetts General Hospital, Boston, MA (USA) we evaluated the performance of basophil activation as a biomarker in monitoring clinical efficacy of oral peanut immunotherapy in a clinical trial.
What is basophil activation?
Basophil activation test is an in vitro challenge test performed in a test tube. Oral food provocation tests (OFPT) are so far the only tool for clinicians to get a one to one response from the patient’s reaction to a suspected food allergen. These OFPT are very complex, especially because intensive care is necessary. These challenges have to be performed in hospitals with the intensive care unit on standby.
Compared to the in vivo challenge the in vitro BAT assay bears much less risk and cost than the intensive OFPT.
What are the results of the research?
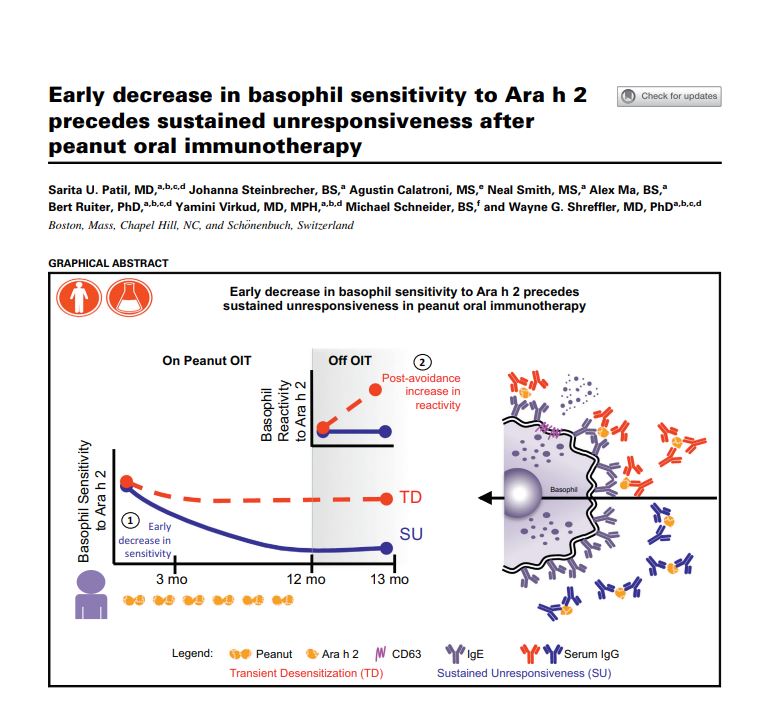
From the clinical point of view, it was again demonstrated, as in many other trials, that only 9 out of 23 allergic patients reached a sustained desensitization while 13 patients showed only transient desensitization which was lost after therapy discontinuation.
From a diagnostic point of view, we were able to monitor and differentiate between those patient groups reaching sustained desensitization and the others. Patients who developed a sustained response showed a significant decrease in basophil sensitivity to the whole peanut protein extract and to one of the main components, Ara h 2.
What does this mean for patients?
One of the main problems in food immunotherapy is that only a portion of the patients develop a clinical response to the therapy. Nevertheless, it is extremely important to offer this treatment to patients since currently, there are no other options to cure food allergies. So, an important goal is to define if a patient will benefit from the treatment or still is at high risk of developing an allergic reaction.
With our work, we could show that monitoring basophil activation has the potential to identify patients showing sustained unresponsiveness during oral immunotherapy. Further, BAT could also indicate when clinical reactivity to peanut allergens might be likely to return in patients after oral immunotherapy.
What’s next?
Our goal is to extend the results of this research to more cases and more therapies in order to offer a clinically validated tool to clinicians and patients.
The immunotherapy itself consists of the concept to treat patients with increasing amounts of the allergen to increase tolerance. Thereby there is a high risk that patients develop a severe clinical reaction. We are evaluating if BAT is able to identify patients at high risk of developing a severe clinical reaction during the immunotherapy treatment. This is still an unmet need in the field of food allergy.
The data presented also showed that basophil sensitivity correlated with severity of clinical reactions. This is another promising indication for using aspects of BAT in oral immunotherapy.
We can conclude that basophil activation is a valid biomarker for sustained clinical efficacy after oral immunotherapy, as well as a marker to evaluate when clinical reactivity to the allergen might be likely to return and a monitoring tool for the efficacy of peanut immunotherapy
About the Interviewee

Related Resources:
Recent Publication
Flow CAST® is for Research Use Only in the US. Not for use in diagnostic procedures.
Health Canada Licence: 101781
CAST® ELISA is for Research Use Only. Not for use in diagnostic procedures.
CAST® allergens are for Research Use Only in the US. Not for use in diagnostic procedures.
Many of BÜHLMANN’s Allergens are Health Canada licensed.




