- Products & Solutions
- FDA IVD
- About Us
- News & Events
- Contact
- Additional Sites
BUHLMANN News
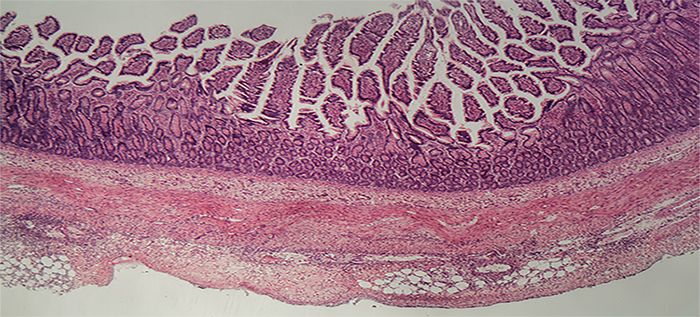
Calprotectin in the Updated ACG Ulcerative Colitis Guidelines
What is Ulcerative colitis? Ulcerative colitis (UC) is a chronic disease affecting the large intestine, in which the lining of ...

Check Us Out at AMLI 2019
We offer a comprehensive autoimmunity product line comprised of robust assays for simple, esoteric testing for important markers and it ...

Visit BUHLMANN at AACC 2019
Assays Offerings: We offer a selection of unique, high-quality assays for Cellular Allergy testing such as the BÜHLMANN Flow CAST® product group and numerous ...

Evaluation BÜHLMANN fCAL® Turbo Test – Kantonsspital St.Gallen
BÜHLMANN fCAL® turbo is for Research Use Only in the US. Not to be used in diagnostic procedures. Health Canada ...

Experience with BUHLMANN fCAL turbo- SYNLAB
BÜHLMANN fCAL® turbo is for Research Use Only in the US. Not to be used in diagnostic procedures. Health Canada ...

BÜHLMANN fCAL® turbo Customer Testimonials
View a compilation of twelve user testimonials from UK, Ireland, Switzerland and France that have been published so far for ...

Educational Webinar- Calprotectin in the Diagnosis of Inflammatory Bowel Disease
Basophils Are Still Alive
Quality Assay & Sample Stability for Viable Basophils Basophil Activation Testing (often referred to as BAT) is a flow-cytometry-based functional ...



