ACE kinetic is FDA 510(k) Exempt. For in vitro Diagnostic Use.
Angiotensin Converting Enzyme (ACE)- Automated Enzymatic Assay – BÜHLMANN ACE Kinetic test
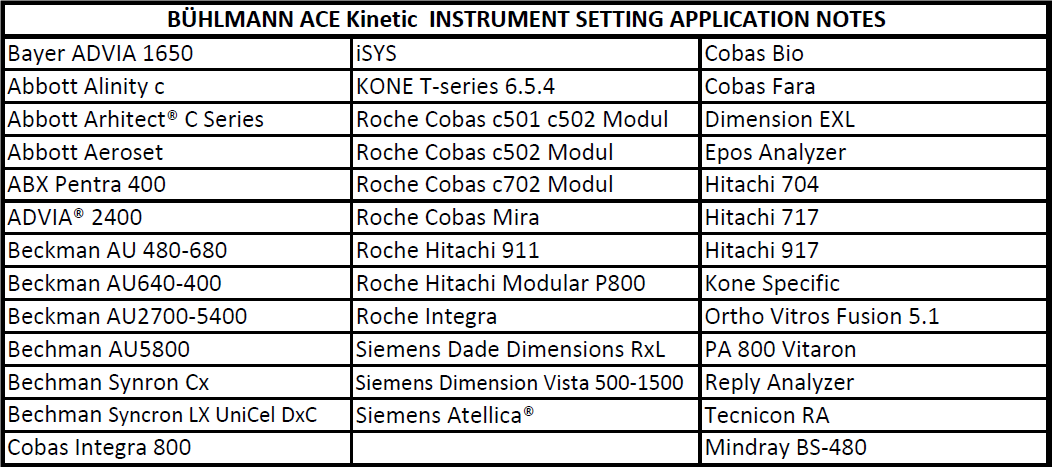
The BÜHLMANN ACE kinetic test is intended for the direct and quantitative in vitro diagnostic determination of angiotensin converting enzyme (ACE) activity in serum by an enzymatic assay. The ACE kinetic kit from BÜHLMANN is a kit providing ready to use Substrate and highly stable Calibrator and Controls, offering a maximum convenience and accuracy. Reconstituted Calibrator and Controls provided with the kits are stable for 6 months at 2-8°C. The substrate has an onboard-stability up to 3 months. Different package sizes are available (2×50, 100 and 1200 tests). Application notes are available on, Beckman AU, Roche Cobas, Siemens Atellica, Abbott Alinity… to name a few, see the full list below.
VIEW PRODUCT HIGHLIGHTS
Angiotensin Converting Enzyme
In vivo, ACE catalyzes the conversion of Angiotensin I to Angiotensin II and inactivates bradykinin during regulating blood pressure via Renin-Angiotensin-System.
Elevated levels of serum ACE have been measured in patients suffering from various disorders. They often indicate a poor prognosis or rapid progression of the disease. Elevated serum ACE levels are reported in granulomatous-inflammatory diseases such as Sarcoidosis and Mixed-Connective-Tissue Disease (MTCD), Nephropathies associated with Diabetes and Glomerulonephritis, Cardiovascular diseases such as left ventricular hypertrophy, brain and myocardial infarction.
The main application is Sarcoidosis. Other areas of application are Chronic Berylliosis and monitoring of compliance under ACE inhibitor therapy.
High Sensitive Methods
In cerebrospinal fluid (CSF) normal ACE activity is far below the detection limit of any commercially available ACE kinetic assay. BÜHLMANN offers a solution to reliably detect even this low ACE activity. The ACE high sensitive assay with a detection limit of 1 U/L.
Product Information
| Method | Enzymatic assay |
| Time to result | 10 min (approx.) |
| Sample type | 25 µl serum |
| Sensitivity | 12 ACE units |
| Catalog Number | see below |
| Status | FDA 510(k) cleared, IVD |
Sarcoidosis
Sarcoidosis is an inflammatory disease of unknown origin characterized by the formation of granulomas. The prevalence of this multisystem granulomatous disorder, which activates the immune system, varies between ethnic groups and geographic regions. In about 20% of the cases especially multi-organ involvement may become life-threatening. The chronic forms can last several years or even a life time.
Consequently, diagnostic tools are essential to closely monitor disease activity and to predict disease progression. ACE is valuable to assess the disease activity, especially in systemic Sarcoidosis. It is released by epithelioid cells and its serum level correlates significantly with the granuloma burden of the patient.
The positive predictive value of elevated ACE activity is estimated between 75-90%, the negative predicted value between 70-80%. An initial low ACE activity indicates a good prognosis.