BÜHLMANN ACE Kinetic Enzymatic Assay
Simple, Fast, Versatile
Automated Assay for use on Multiple Clinical Chemistry Platforms
ACE kinetic is FDA 510(k) cleared. For in vitro Diagnostic Use.
BÜHLMANN’s Clinical Chemistry product line is a unique selection of high quality assays for routine and specialized testing in clinical chemistry. Employing the long lasting experience with ACE activity on a broad range of clinical chemistry analyzers BÜHLMANN focuses on continuing to expand the existing product line with either specialized niche products or new upcoming markers for routine applications.
BÜHLMANN ACE Kinetic Enzymatic Assay is one of the top assays in the Clinical Chemistry product line and it is designed for direct and quantitative determination of Angiotensin Converting Enzyme (ACE) activity. The ACE kinetic assay allows clinical routine laboratories the ability to measure enzyme activity in serum with an easy to use procedure for use on most open clinical chemistry platforms. Validated and IVD marked protocols are provided with a complete reagent package including calibrator and controls.
Why Measure ACE?
Elevated levels of serum ACE have been measured in patients suffering from various disorders as they often indicate a poor prognosis or rapid progression of the disease:
- Granulomatous-inflammatory diseases such as:
- Sarcoidosis
- Chronic Berylliosis
- Mixed-Connective-Tissue Disease (MTCD)
- Nephropathies associated with
- Diabetes
- Glomerulonephritis
- Cardiovascular diseases such as:
- Left ventricular hypertrophy
- Brain and myocardial infarction
Other Reasons to Measure:
Monitoring of ACE inhibitor therapy
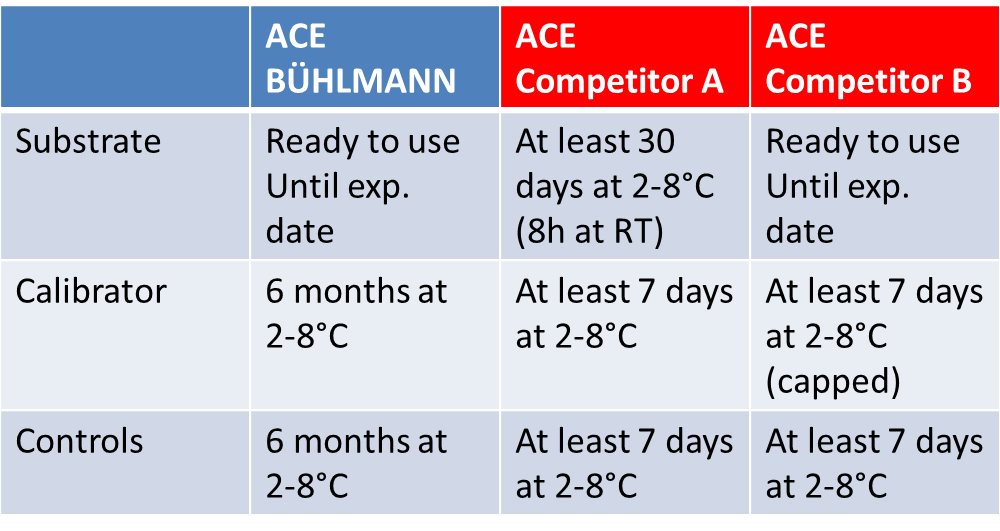
Advantages of BÜHLMANN ACE Kinetic Assay
Disadvantages of Competitors’ ACE Assays
Validated Applications
Abbott Aeroset
Abbott Arhitect C Series
ABX Pentra 400
Bayer Advia 1650
Beckman AU480/680
Beckman AU640/400
Beckman AU2700/5400
Beckman AU5800
Beckman Synchron Cx
Beckman Synchron Lx /UniCel DxC
Kone T- series
Roche Hitachi 911
Roche Cobas Integra 800
iSYS
KONE T-series 6.5.4
Roche Cobas c501 c502 Modul
Roche Cobas c502 Modul
Roche Cobas c702 Modul
Roche Cobas Mira
Roche Hitachi 911
Roche Hitachi Modular P800
Roche Integra
Siemens Advia 2400
Siemens Dade Dimensions RxL
Siemens Dimension Vista 500-1500
Cobas Bio
Cobas Fara
Hitachi 704
Hitachi 717
Hitachi 917
Kone Specific
Ortho Vitros Fusion 5.1
PA 800 Vitaron
Reply Analyzer
Tecnicon RA
Under Validation:
Advia 1800
Comparison Data: Stability of Reagents after Reconstitution