
BÜHLMANN fCAL® turbo is for Research Use Only in the US. Not to be used in diagnostic procedures.
Health Canada License: 96808
A Report of Martina Kobelt* and Karin Jung**, Zentrum für Labormedizin, KS St. Gallen, Switzerland
*Senior Laboratory Technician Core Lab, **Deputy Head of Department Core Lab/FAMH Candidate Clinical Chemistry, Centre for Laboratory Medicine, Frohbergstrasse 3, 9001 St. Gallen, Switzerland
Centre for Laboratory Medicine
The Centre for Laboratory Medicine at the cantonal hospital of St. Gallen has offered fecal calprotectin testing for many years. In the past, fecal calprotectin samples were analysed in batches once or twice a week using an ELISA test.
Our goal was to set up an automated random access fecal calprotectin testing in order to be able to analyse and report results on a daily base and give a faster service for our clinicians.
For us, it was important to introduce a test which corresponds to the well-established clinical cut-off values used before.
In the ELISA we had already applied a one-point calibration proposed by the manufacture to reduce costs, so cost efficiency of the new assay was also very important.
Installation and adaptation of fCAL® turbo
The BÜHLMANN fCAL® turbo assay was an ideal candidate to fulfil these expectations. The adaptation of the BÜHLMANN fCAL® turbo on the DxC 800 analyser from Beckman Coulter was very easy and fast. We set up the protocol within a few hours, and the handling of the new method is comparable to any other clinical chemistry assay.
Our initial concerns about potential contamination and carry-over, from analysing fecal samples on chemistry analyser were dispelled by the detailed information BÜHLMANN provided. After using the BÜHLMANN fCAL® turbo for nearly one year no negative observation were made.
During the method validation we observed a non-linear correlation to the ELISA at samples higher than 300 µg/g i.e. ELISA results were significantly lower than the turbidimetric results. The very good linearity of the fCAL turbo in a range from 20 to 8000 µg/g was highly convincing and consequently allowed us to minimize retesting of additionally diluted samples.
Benefits of the new assay
After the successful evaluation, we informed our clinicians about the introduction of a new test method for analysing fecal calprotectin levels. Since the BÜHLMANN fCAL® turbo showed identical cut-off values compared to the established ELISA assay, our clinicians didn`t need to change the cut-off levels and had the additional benefit of a much higher test range up to 8000µg/g using the new assay. So far, we haven’t been contacted by our clinicians regarding suspicious test results.
The adaptation of the BÜHLMANN fCAL® turbo in our laboratory resulted in many benefits. First, the assay allows us to measure calprotectin levels on a daily base and deliver test results on the same day. This improves our service tremendously, which could not have been managed when using the ELISA test method.
Secondly, the BÜHLMANN fCAL® turbo has a huge test range and shows an excellent linearity from 20 to 8000 µg/g. This results in less sample dilutions and therefore in less workload.
Finally, the calibration is stable for 30 days saving time and money.
We have also optimized our workflow tremendously in regard of fecal sample extraction. Extraction is done in the microbiology laboratory. Before, incoming samples were collected and stored frozen until extraction was started several days later. This meant thawing the samples first which was time consuming, too.
Extraction with the CALEX® Cap
Today, incoming samples are extracted with the CALEX® Cap extraction device and tested all in the same day.
When the fecal sample is completely dispersed in extraction buffer, a short centrifugation is needed and then we can put the CALEX® Cap directly onto the DxC system. No additional transfer of the sample into new tubes or dilution step is needed anymore.
The BÜHLMANN fCAL® turbo test is a reliable, fast and easy automated method delivering results within 10 minutes. We can highly recommend the use of BÜHLMANN fCAL® turbo and are completely satisfied with the service and support of BÜHLMANN, especially during the set-up of the automated protocol. BÜHLMANN helped us with their problem solving abilities; in addition, we appreciate the cooperation between Beckman Coulter and BÜHLMANN.
*This interview was shortened and edited for a better overview
The handling of the new method is comparable to any other clinical chemistry assay.
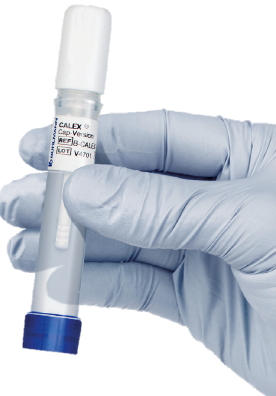
“Incoming samples are extracted with the CALEX® Cap extraction device and tested all in the same day.




