Advancing Medical Laboratory Immunology
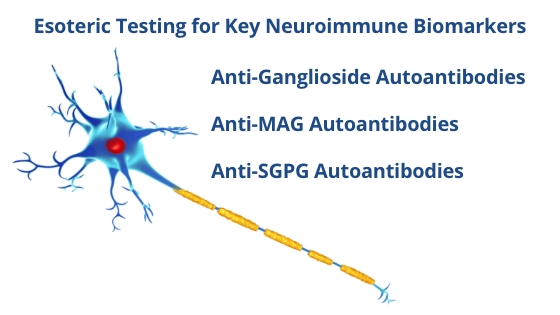
BUHLMANN is proud to partner with AMLI 2025 and support the advancement of medical laboratory immunology through our specialized assay portfolio. We are particularly excited to highlight our expanding line of neuroimmunology tools designed to support research in complex neurological and autoimmune conditions—offered as Research Use Only (RUO) assays and backed by a strong foundation of scientific publications.
Additionally, we will showcase our FDA-cleared calprotectin assays for detecting intestinal inflammation—offering a reliable tool to help distinguish inflammatory bowel disease (IBD) from functional disorders like IBS. Our pancreatic elastase test provides a non-invasive method for assessing exocrine pancreatic insufficiency, supporting digestive health diagnostics. BUHLMANN’s Basophil Activation Test (BAT) for allergy research, available as a Research Use Only (RUO) assay, further reflects our commitment to delivering high-quality solutions for specialized testing needs.
Whether you’re focused on clinical diagnostics or advanced immunology research, our team is eager to discuss how BUHLMANN’s innovative assays can support your lab’s goals.
RUO Solutions
US: BÜHLMANN Flow CAST® and Neuroimmunology Assays are for Research Use Only. Not for use in diagnostic procedures.
EU: All BÜHLMANN products listed below are IVDR compliant (excluding Anti-SGPG Autoantibodies).
IVD Assays
BÜHLMANN fCAL® turbo

BÜHLMANN fCAL® turbo is a fully automated high-throughput calprotectin assay
US: BÜHLMANN fCAL® turbo (K190784): FDA 510(k) cleared. For in vitro Diagnostic Use.BÜHLMANN fCAL® turbo + CALEX® Cap (K191718 & K232057): FDA 510(k) cleared. For in vitro Diagnostic Use. CPT Code: Calprotectin – 83993.
EU: BÜHLMANN fCAL® turbo is IVDR compliant.
BÜHLMANN fPELA® turbo

BÜHLMANN fPELA® turbo is a fully automated, high-throughput pancreatic elastase assay:
US: BÜHLMANN fPELA® turbo: FDA 510(k) Exempt. For in vitro Diagnostic Use. CPT Code: Pancreatic Elastase– 123234
EU: BÜHLMANN fPELA® turbo is IVDR compliant.
CALEX® Cap
CALEX® Cap Stool Preparation Device for fecal calprotectin & fecal pancreatic elastase
US: BÜHLMANN fCAL® turbo + CALEX® Cap (K191718 & K232057): FDA 510(k) cleared. For in vitro Diagnostic Use.
EU: IVDR compliant.
Pair with BÜHLMANN fCAL® turbo for a highly sensitive and reliable solution for detecting gastrointestinal inflammation.
At the heart of this system is the CALEX® Effect – a unique extraction process, enabled by the CALEX® Cap Device, which combines synergistic buffer components and a patented dilution, to maximize calprotectin recovery and stability to help reduce the chance of missed inflammatory signals.

The CALEX® Effect enables optimal extraction, which elevates the diagnostic potential of the entire testing system by delivering unmatched efficiency and analyte stability.*
*Calprotectin extracts are stable at room temperature for up to 2 days and up to 15 days at 2-8°C. Pancreatic elastase extracts are stable at room temperature for 8 days and up to 12 days at 2-8°C.
Venue/ Contact Information
Contact BUHLMANN AMLI Team for general questions or schedule a meeting ahead of time!
Connect with us at AMLI 2025 – Booth 24
August 14-17, 2025 | EXPLORE THE PROGRAM
Hyatt Regency Minneapolis
Minneapolis, MN, USA