BÜHLMANN fCAL® turbo + CALEX® Cap (K191718 & K232057): FDA 510(k) cleared. For in vitro Diagnostic Use.
BÜHLMANN fPELA® turbo is FDA 510(k) Exempt. For in vitro Diagnostic Use.
CALEX® Cap Updates
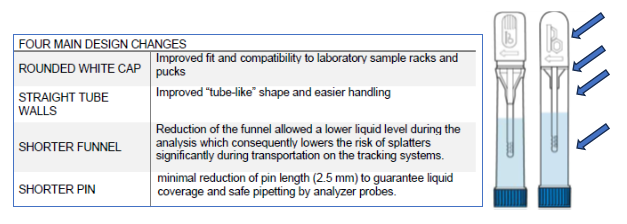
CALEX Cap 4.0- Improved Total Laboratory Automation (TLA), or Block Automation & Seamless Integration
- TLA Compatibility: CALEX®Cap 4.0 integrates smoothly with TLA, enhancing workflow efficiency on track systems.
- Block Automation: no track, CALEX® Cap 4.0 integrates with decapping units to mitigate repetitive motion injuries and keep lab techs happy.
- New LONGER EXTRACT Stability!
- Special 510(k) clearance for CALEX® Cap (K232057) with fCAL® turbo, which extends the extraction stability to 2 days @ RT and 15 days @ 2-8°C.
- Seamless Integration: CALEX® Cap 4.0 improves stool sample preparation without changing procedure (no re-validation required), meeting TLA requirements seamlessly.
- Learn how CALEX® Cap Streamlines Workflow for Calprotectin testing with fCAL® turbo and fPELA® turbo in this video- See it in action






