BÜHLMANN ACE Kinetic Enzymatic Assay
Unique in Speed, Quality, Flexibility
Automated Assay for use on Multiple Clinical Chemistry Platforms
ACE kinetic is FDA 510(k) Exempt. For in vitro Diagnostic Use.
Overview
BÜHLMANN’s Clinical Chemistry product line offers a unique selection of high-quality assays for both routine and specialized clinical chemistry testing. With extensive expertise in ACE activity measurement across a broad range of clinical chemistry analyzers, BÜHLMANN ensures reliability, reproducibility, and accuracy—delivering precise, trustworthy results.
BÜHLMANN ACE Kinetic Enzymatic Assay is designed for direct and quantitative determination of Angiotensin-Converting Enzyme (ACE) activity. This assay allows clinical routine laboratories to measure enzyme activity in serum with an easy-to-use procedure compatible with most open clinical chemistry platforms. With over 20 application notes, the assay comes as a complete reagent package, including a calibrator and controls.
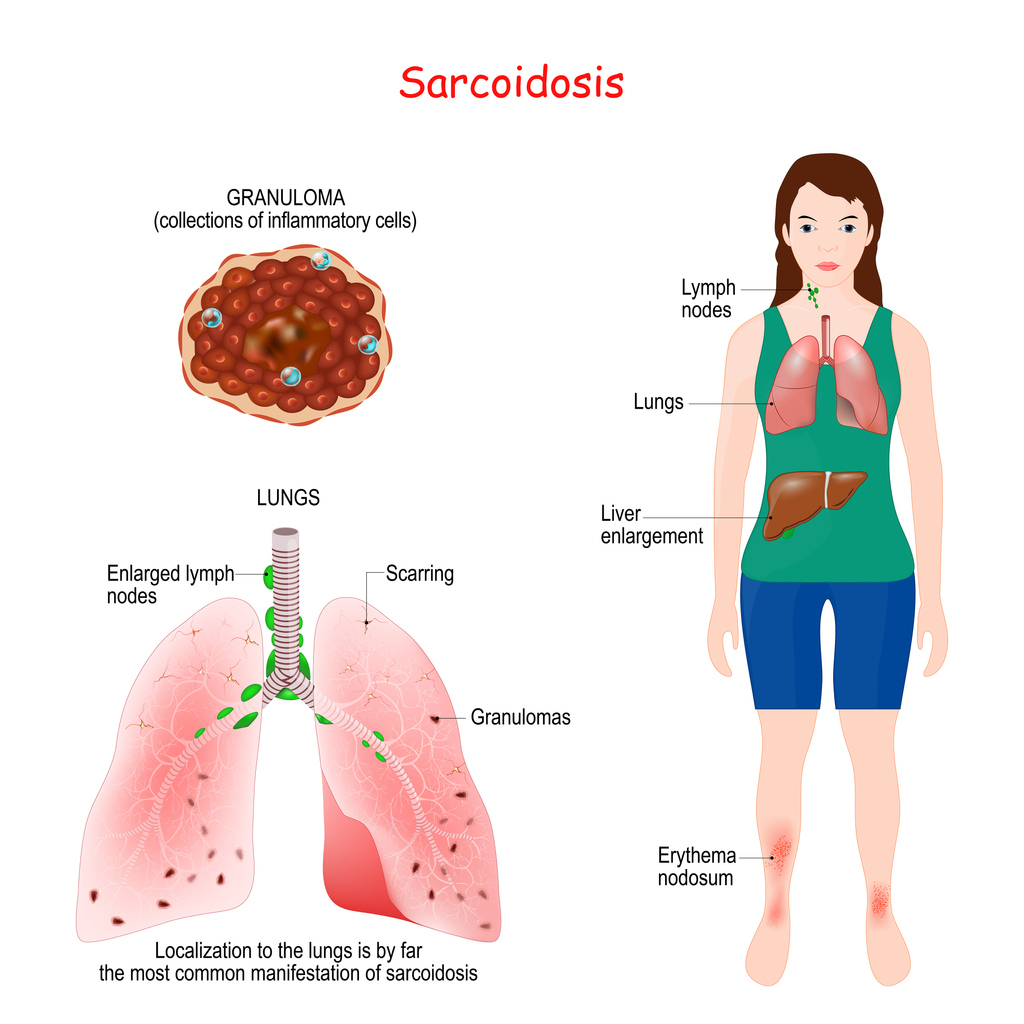
What is Sarcoidosis?
Sarcoidosis is a disease in which inflammation produces tiny lumps of cells in organs throughout the body. The lumps, called granulomas, most often appear in the lungs but can also occur in the lymph nodes, eyes, skin, or other areas of the body.[1]
While many patients experience mild or self-limiting symptoms, others may develop chronic or progressive disease that can lead to severe complications, including pulmonary fibrosis and organ dysfunction.2 Due to its unpredictable nature and potential for multi-organ involvement, early detection and accurate assessment of disease activity are crucial in guiding treatment decisions and preventing long-term damage.3 Elevated ACE levels are commonly used to assess disease activity, as granulomas produce ACE and reflect the level of inflammation associated with sarcoidosis.4
Why Measure ACE?
ACE is an enzyme that regulates blood pressure. In sarcoidosis, however, it is often elevated due to the increased activity of immune cells within granulomas.1,5,6 In clinical settings, assays such as the BÜHLMANN ACE kinetic assay are commonly used for rapid and reliable measurement of serum ACE, supporting their relevance in routine assessment of disease activity in patients with sarcoidosis in conjunction with other clinical and laboratory findings. 5,6
BÜHLMANN ACE Kinetic Enzymatic Assay – See the Difference
Advantages of BÜHLMANN ACE Kinetic Assay
Speed
Optimized for Faster Implementation
Pre-optimized protocols enable fast lab implementation & efficient throughput
Quality
Excellent Reagent Stability
Enhanced onboard stability (see below)
Reproducibility, Repeatability, & Precision
Delivers reliable, accurate results
Complete Kit
All reagents available in one complete kit, offered in various kit sizes: 100 tests, 2 x 50 tests, 400 tests, 1200 tests
Flexibility
>20 Application notes
Can be used on most clinical chemistry platforms (see below)
Disadvantages of Competitors’ ACE Assays
–
Potential for Longer Setup or Customization
May require internal validation or workflow adjustments depending on platform.
–
Limited Stability of Reagents
Short expiration dates
Inconsistent Results & High Variability
Poor lot-to-lot reproducibility leading to unreliable and fluctuating data
Single Reagent Set
All reagents only sold separately, not available as a complete kit
–
Limited Validated Applications
Limited chemistry platform compatibility
CLIA Approved Application Notes
| CLIA Approved Application Notes | |
|---|---|
| Manufacturer | Analyzer |
| The Binding Site | Optilite |
| Roche | cobas c701 |
| cobas c702 | |
| cobas c501 | |
| cobas c502 | |
| cobas c303 | |
| Mindray | BS-480 |
| Abbott | Alinity c System |
| Architect c4000 | |
| Architect c8000 | |
| Architect c16000 | |
| Beckmann Coulter | AU5800 |
| Siemens | Attellica CH |
| Thermo Fisher | Indiko |
| Indiko Plus | |
| Pending: Roche c503, c503 & Beckman: AU480-AU680-DxC700AU combo | |
Please contact BUHLMANN Diagnostics Corp for a complete list of validated applications. Contact Sales for more information.
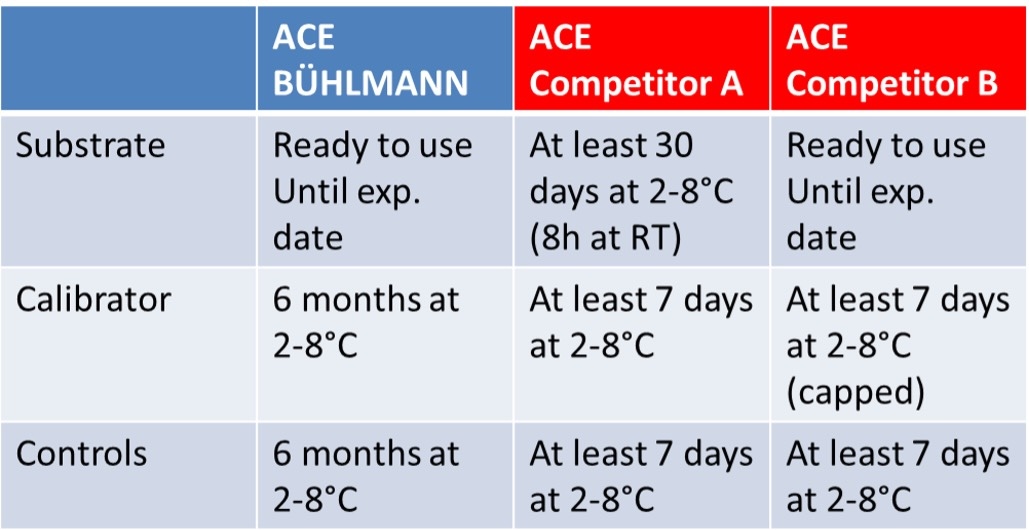
Comparison Data: Stability of Reagents after Reconstitution
Enhancing Diagnostic Confidence
With sarcoidosis affecting an estimated >202,000 individuals in the U.S.,[2] having a reliable ACE test is critical. The BUHLMANN ACE Kinetic Assay provides clinicians with a robust, efficient, and clinically validated solution for assessing ACE levels as an aid in disease activity evaluation.
[1] American Thoracic Society. “What Is Sarcoidosis?” American Thoracic Society, n.d., https://www.thoracic.org/patients/patient-resources/resources/what-is-sarcoidosis.pdf.
[2] Baughman, Robert P., et al. “Pulmonary Sarcoidosis: A Comprehensive Review: Past to Present.” Clinics in Chest Medicine, vol. 44, no. 4, Dec. 2023, pp. 609–624. https://www.sciencedirect.com/science/article/pii/S0896841123001166.
[3] Melani, Andrea S et al. “A Comprehensive Review of Sarcoidosis Diagnosis and Monitoring for the Pulmonologist.” Pulmonary therapy vol. 7,2 (2021): 309-324. doi:10.1007/s41030-021-00161-w.
[4] Kawai, Hideki et al. “Serum angiotensin-converting enzyme levels indicating early sarcoidosis diagnosis and immunosuppressive therapy efficacy.” ESC heart failure vol. 10,3 (2023): 1803-1810. doi:10.1002/ehf2.14343.
[5] BUHLMANN Laboratories. ACE Kinetic Assay Instructions for Use, US version, BUHLMANN Laboratories AG 2023.
[6] Seedahmed, Mohamed I., et al. “Epidemiology of Sarcoidosis in U.S. Veterans From 2003 to 2019.” Annals of the American Thoracic Society, vol. 20, no. 6, Feb. 2023, pp. 797–806. https://doi.org/10.1513/annalsats.202206-515oc.