Key Highlights
We are thrilled to share the highlights of our successful participation in the Association for Diagnostics and Laboratory Medicine (ADLM) 2024 Annual Meeting & Clinical Lab Expo, held from July 28 to August 1 in Chicago, IL.
ADLM 2024 Assay Offerings
Our booth showcased our extensive range of fecal calprotectin (fCAL) products, the broadest line in the industry, available in the United States. These include both FDA-cleared and Research Use Only (RUO) kits.
Product Showcased:
- FDA 510(k) cleared: BÜHLMANN fCAL® ELISA, BÜHLMANN fCAL® turbo, and CALEX® Cap
- FDA exempt: BÜHLMANN fPELA® turbo (Pancreatic Elastase)
Other RUO Assays Showcased:
- Quantum Blue® fCAL Rapid Tests
- Quantum Blue® TDM and Antibody Rapid Tests
- Cellular Allergy (Flow CAST®)
- Neuroimmunology Assays (MAG, SGPG, and Gangliosides)
Poster A-502
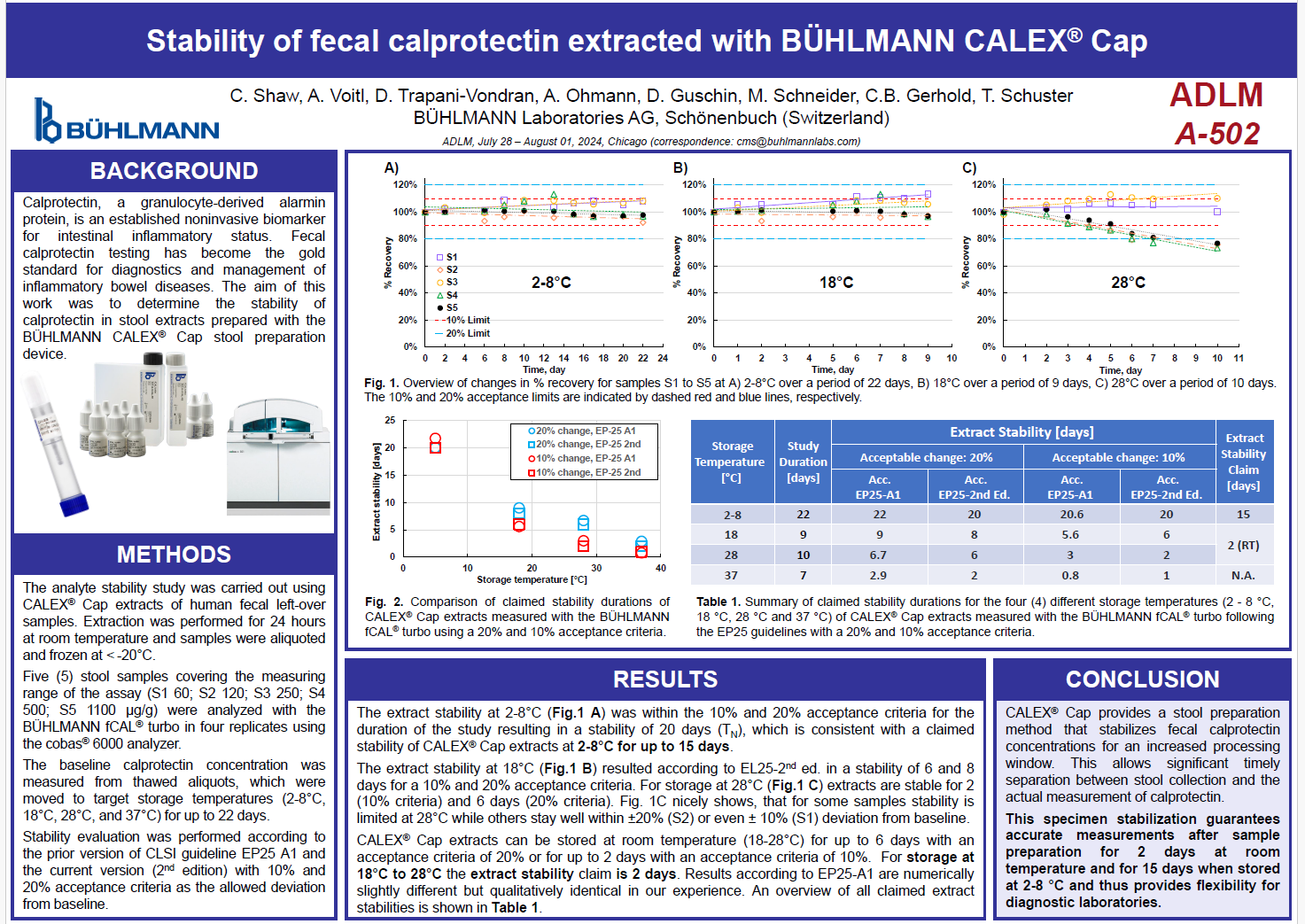
Stability of fecal calprotectin extracted with BÜHLMANN CALEX® Cap
BÜHLMANN presented a study at ADLM 2024 on the stability of fecal calprotectin in stool extracts prepared using the CALEX® Cap stool preparation device.
For more details, you can view our poster HERE.

Looking Ahead:
As we reflect on ADLM 2024’s success, we are more committed than ever to driving innovation in diagnostic testing. Stay tuned for more updates on our upcoming projects and product launches.
Thank you to everyone who visited our booth and checked out our poster presentation. We look forward to seeing you at ADLM 2025!