
Quality Assay & Sample Stability for Viable Basophils
Basophil Activation Testing (often referred to as BAT) is a flow-cytometry-based functional assay that assesses the degree of cell activation after exposure to stimuli.3
A: If you are considering either of the BAT applications mentioned above, you may need to answer critical questions, such as: how stable are basophils after the blood collection and which anticoagulant is appropriate to preserve viability?
If you search literature on the internet it will be difficult to retrieve precise and conclusive information regarding the acceptable timeframe for testing post-venipuncture. In fact, you will find conflicting, confusing, and misleading data that may give the perception that basophils are very delicate cells that require testing as quickly as possible. It’s not uncommon to find statements like “BAT test must be performed immediately after venipuncture” or “Whole blood BAT should ideally be performed within 4 h of blood collection to maximize viability and functionality of basophils1, but these statements are not accompanied by a reference to a clear analytical evaluation.
About the Author

Michele Romano, PhD
Michele Romano, PhD, is responsible at BÜHLMANN for the Product Management of the CAST® Assays for Basophil Activation Tests (BAT) in the field of in vitro allergy diagnosis as well as in the promising new application of drug candidate potency and efficacy testing.
He received his PhD degree in Cellular and Molecular Biology and Pathology at University of Verona, Italy and a degree in Industrial Biotechnology at University of Milano, Italy. He has joined BÜHLMANN in 2011, after a research career in the immunology field both in Industry and Academia, with the main focus in functional bioassay development and immune receptor characterization.
BÜHLMANN has been at the forefront of basophil activation test development for over 20 years, combining innovation with commitment to high quality standards. The first assay was launched soon after Edward Knols’ discovery of CD63 activation marker2 and next generation assays evolved thereafter following the first BAT publication.4 Please see the full assay evolution in timeline below.

Results
The results obtained were quite clear and comprehensive. In fact, we found that viable basophils can be recovered not only immediately after blood collection but also over several days in any anticoagulant used. However, the optimal efficiency of recovery was seen with EDTA blood stored at 2-8°C. At this temperature, the loss of cells is minimized, with recovery of more than 500 basophils, which is the threshold to obtain a statistically significant number of cells for robust BAT data. EDTA blood stored at 2-8°C was superior to heparin and citrate blood, where we only recovered a median of basophils between 300 and 500.
In terms of activation capabilities, basophils responded well to different stimuli. We confirmed the basophil activation by cross-linking the high affinity receptor for the IgE with a monoclonal antibody (the positive control included in any of our kits (anti FceRI Ab)) and several allergens.
We built very nice dose response curves, with a minimal loss of signal up to 24 hours after blood collection, slight increase at 48 hours but still making it feasible to obtain high quality BAT data (See Figure 1 below).
Dose Response Curves
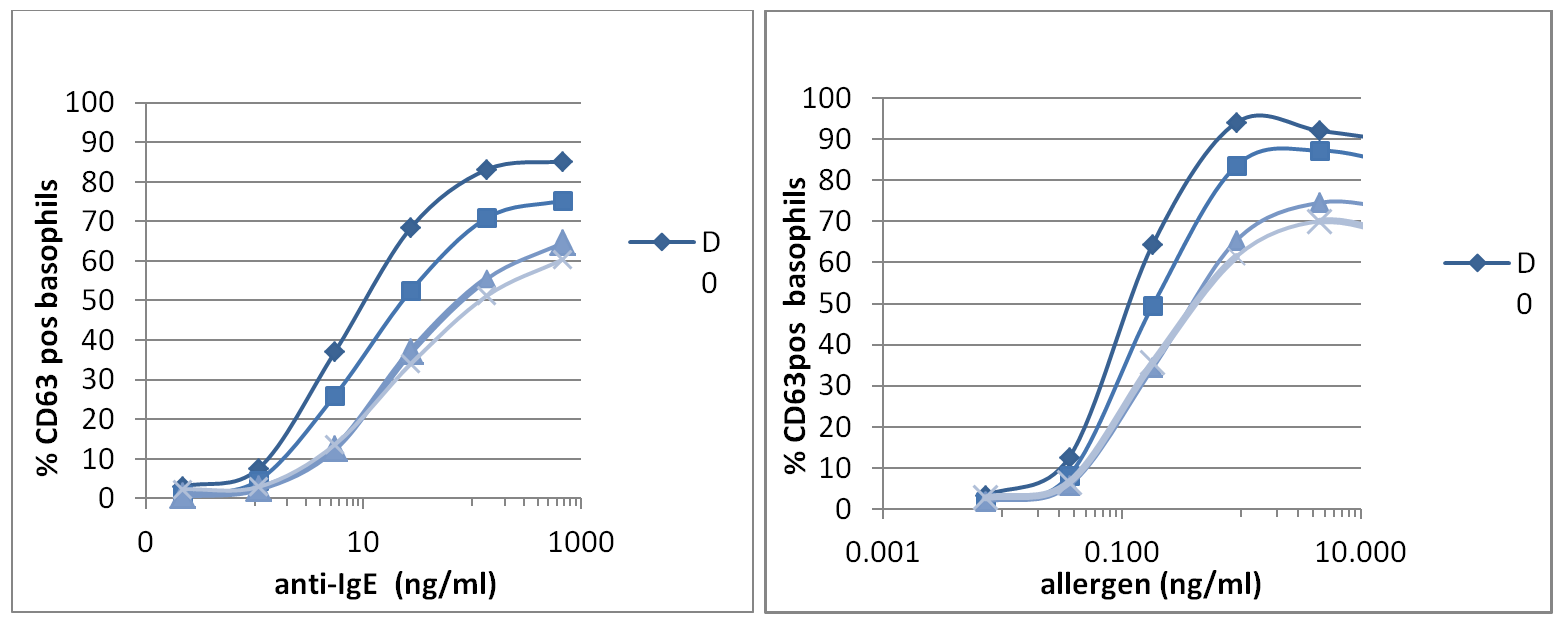
Figure 1: Representative case of EDTA blood donors analysed with Flow CAST® for CD63 activation over a period of 3 day storage at 2-8° by anti FceRI Ab (left) and specific allergen (right). D0, D1, D2, D3 is representative of the days after venipuncture.
Conclusion:
Our results suggest that whatever application of basophil activation is used, the cells are viable for up to 24/48 hours after blood collection, for drug and protein allergens, respectively.
We have reported the robustness of the BAT data in conjunction with time of storage demonstrating its dependence on the allergen used (You can find this information in the IFU of our CAST® assays and in the allergen booklets). For example, protein allergen typically gives very high responses, so BAT can be performed up to 48 hours after blood collection, since the loss of activation does not affect the results. Alternatively, drug allergens rarely give activations that exceed 20-30%, so we suggest to perform BAT testing within 24 hours to limit the potential of loss of reactivity and the generation of false negative results.
At the same time, when using the basophil sensitivity as a parameter of BAT, intended as the EC50 (Half maximal effective concentration of the allergen) of a dose response curve of your stimuli, you should take into account the limited variability in your data generated by the storage of blood.
Of course working with live cells is not the same as working with serum analytes, but the timeframe to obtain high quality data from a BAT test works well with sophisticated sample logistics of diagnostic labs. Therefore, a standardized basophil activation test can be successfully implemented in diagnostic labs as well as in is widely used in research.
If you want to obtain more details on this data, please contact us for list of additional resources. These resources will provide information on the stability of CD63 activation in different conditions (ie. anticoagulant, storage temperature, and stimulation buffer) as well as differences with other activation markers such as CD203c.
Flow CAST® is for Research Use Only in the US. Not for use in diagnostic procedures.
Health Canada Licence: 101781
CAST® ELISA is for Research Use Only. Not for use in diagnostic procedures.
CAST® allergens are for Research Use Only in the US. Not for use in diagnostic procedures.
Many of BÜHLMANN’s Allergens are Health Canada licensed.
References
- Hemmings, O., Kwok, M., McKendry, R. et al. Curr Allergy Asthma Rep (2018) 18: 77. 1007/s11882-018-0831-5
- Knol EF, Mul FP, Jansen H, Calafat J, Roos D. Monitoring human basophil activation via CD63 monoclonal antibody 435. J Allergy Clin Immunol 1991;88:328–338.
- McGowan, Emily C and Sarbjit Saini. “Update on the performance and application of basophil activation tests” Current allergy and asthma reports 13,1 (2013): 101-9. doi: 10.1007/s11882-012-0324-x PMID: 23188565.
- Sainte-Laudy J, Vallon C, Gu[1]erin JC. Analysis of membrane expression of the CD63 human basophil activation marker. Applications to allergologic diagnosis. Allerg Immunol (Leipz) 1994;26:211–214.
- Santos AF, Shreffler WG. Road map for the clinical application of the basophil activation test in food allergy. Clin Exp Allergy. 2017 Sep;47(9):1115-1124. doi: 10.1111/cea.12964. Epub 2017 Aug 1. Review. PubMed PMID: 28618090 DOI: 1111/cea.12964.




