Visit our booth to get an update on our Calprotectin and Therapeutic Drug Monitoring product portfolio:
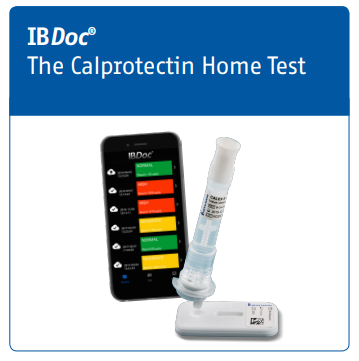
IBDoc®, the first in-vitro diagnostic POC and home testing device to measure the inflammatory marker, fecal calprotectin.
BÜHLMANN IBDoc® is an in vitro diagnostic immunoassay for the quantitative determination of fecal calprotectin in human stool. The results of the assay are analyzed by a downloadable smartphone application. IBDoc® is intended as an aid to the determination of the inflammatory status of the patient’s intestinal mucosa for inflammatory bowel disease (e.g. Crohn’s Disease and Ulcerative Colitis) monitoring. IBDoc® is an assay developed for self-testing / home use by trained patients ages 12 and above that are under the care of a healthcare practitioner. The test may also be used in a near-patient or laboratory setting.
Health Canada Licence: 98903, Device class: 3
BÜHLMANN IBDoc® is not available for sale in the US.

Quantum Blue® platform offering rapid and quantitative results for serum calprotectin, CRP, serum infliximab trough levels, and serum adalimumab trough levels (Additional trough level assays coming soon!)
Health Canada Licenses:
Quantum Blue® Infliximab: 98838
Quantum Blue® Adalimumab: 101776
Quantum Blue®Infliximab and Quantum Blue® Adalimumab is for Research Use Only in the US. Not for use in diagnostic procedures.
COLLIN SHAW
VP of Business Development





